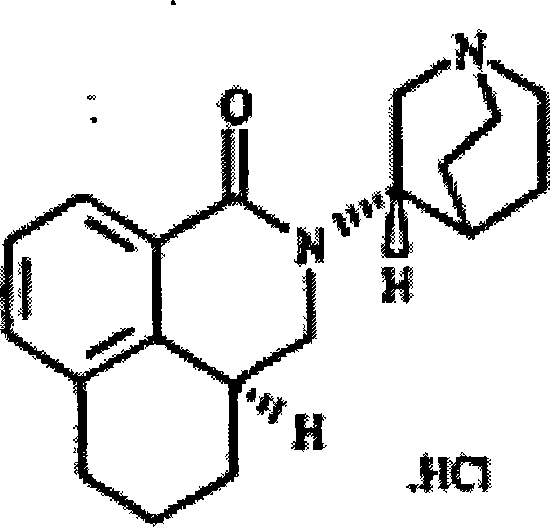
Production technique of hydrochloric acid palonosetron
A technology of palonosetron and hydrochloric acid, which is applied in the field of new production technology, can solve the problems of product yield reduction, difficulty, and purity difficulty, and achieve the effects of improving product quality, simplifying operation steps, and optimizing technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
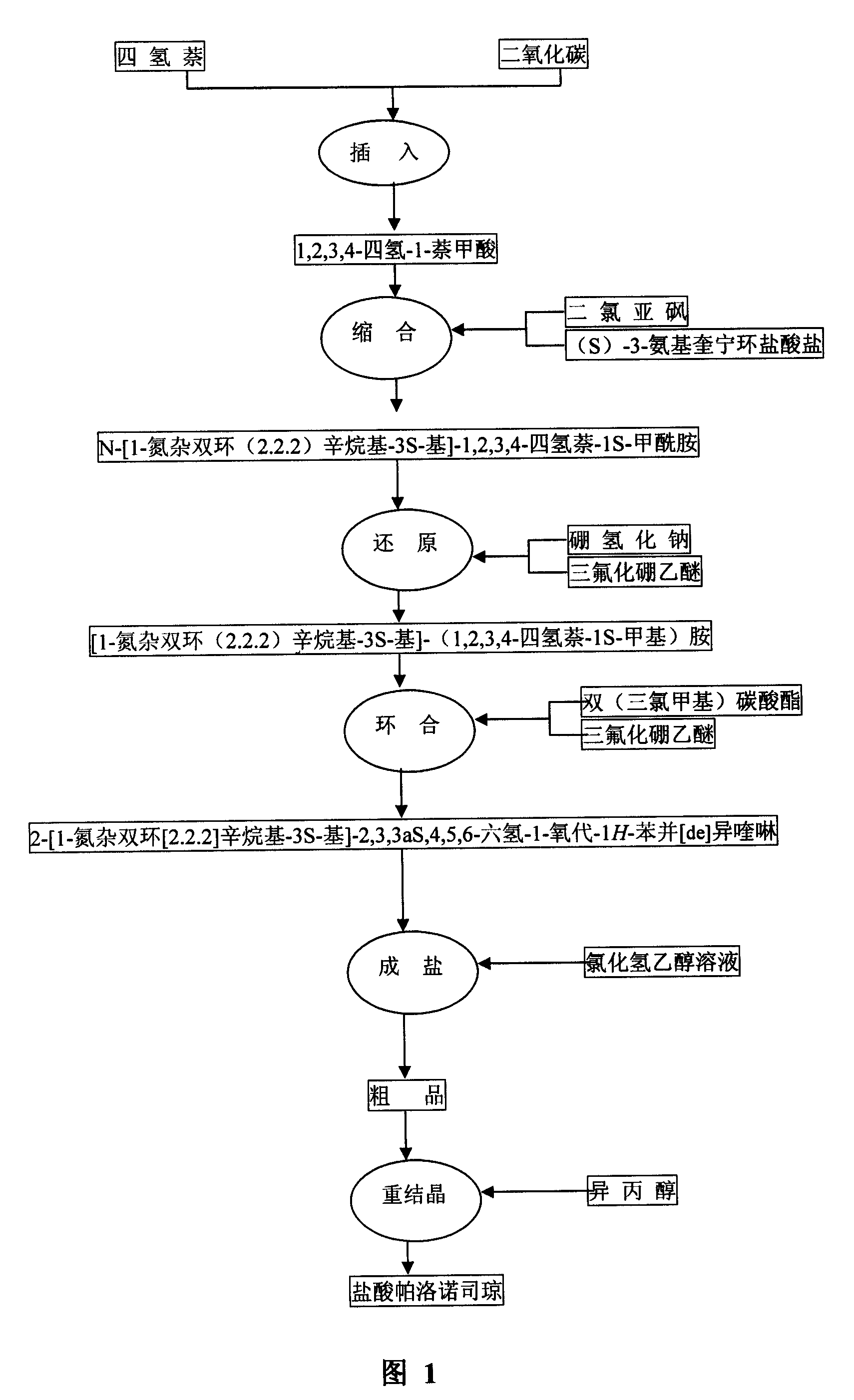
[0027] Example 1 1,2,3,4-tetrahydro-1-naphthoic acid preparation
[0028] ①Reaction formula:
[0029]
[0030] ②Operation method:
[0031] In a 250ml three-neck flask, add 7.4g (66mmol) potassium tert-butoxide, vacuumize, and blow nitrogen three times. Then add 170ml of n-hexane and 7.8ml (52mmol) tetramethylbutanediamine with a needle tube, cool to -30°C, stir for 10 minutes, slowly add 33ml of n-butyllithium with a needle tube, after the dropwise addition is complete, stir for another 10 minutes , add 8.5ml (63mmol) tetrahydronaphthalene, stir at -30°C for 30 minutes, then naturally warm up to room temperature, feed carbon dioxide for 30 minutes, then slowly add 120ml of water, separate layers, take the water layer and acidify it with hydrochloric acid, and use Extracted with ethyl acetate three times, combined the ethyl acetate layers, washed with water, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and recovered the solvent under...
Embodiment 2
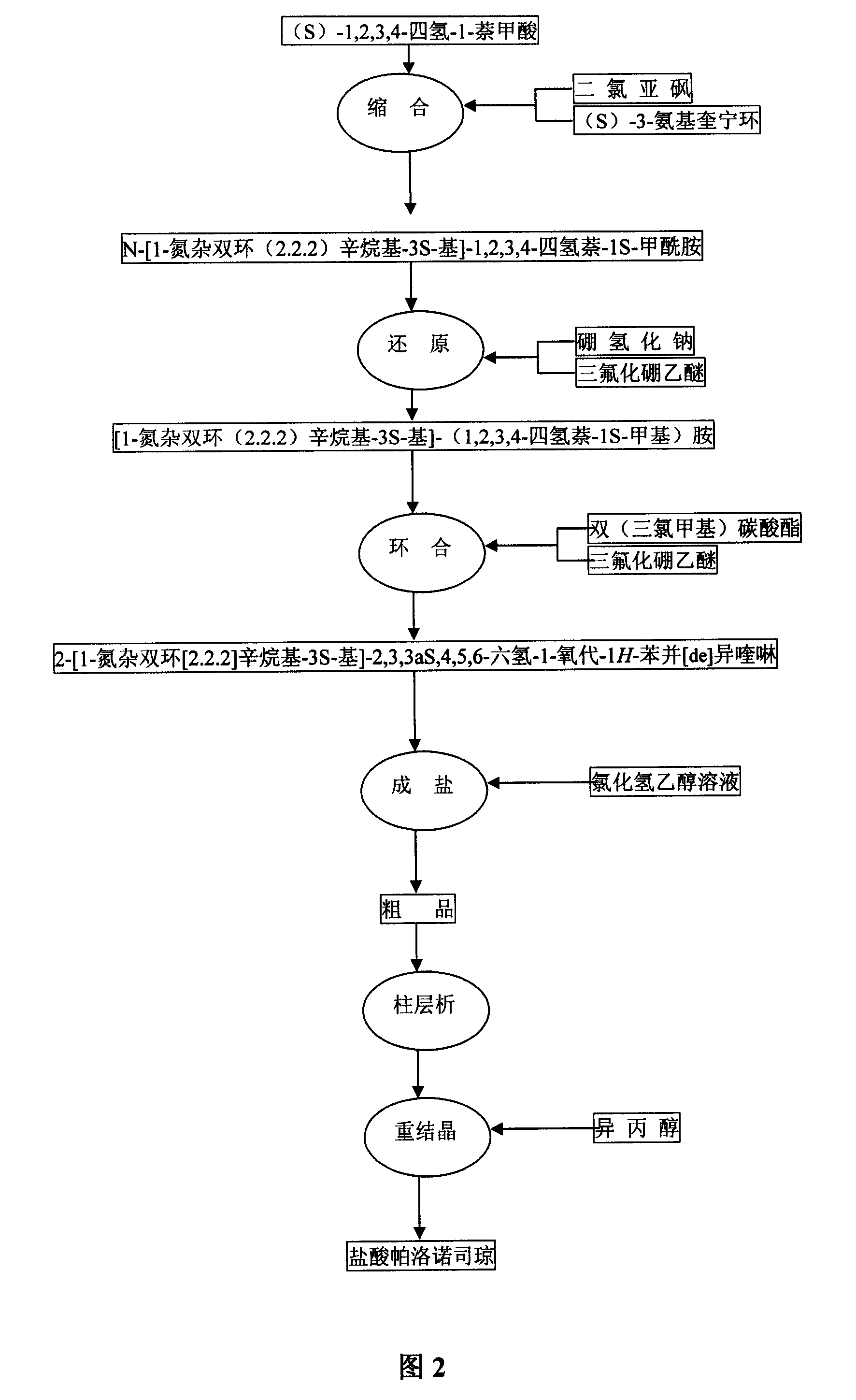
[0032] Example 2 Preparation of N-[1-azabicyclo(2.2.2)octyl-3S-yl]-1,2,3,4-tetrahydronaphthalene-1S-carboxamide
[0033] ①Reaction formula:
[0034]
[0035] ②Operation method:
[0036]In a 100ml three-neck flask, add 5.81g (33mmol) of compound and 50ml of toluene, and then add 12ml of thionyl chloride. Heat to reflux and stir the reaction at reflux for 1 hour. Reaction complete, distill off most of toluene, remaining toluene solution is dripped slowly in the solution (80ml) of ethyl acetate that is dissolved with 5.97g (S)-3-aminoquinuclidine hydrochloride (30mmol), The reaction was continued for 3 hours at room temperature. After the reaction was completed, 50% sodium hydroxide solution was added to adjust the pH to alkaline. Then extract twice with ethyl acetate, combine the ethyl acetate layers, wash once with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and recover the solvent under reduced pressure to obtain a crude product, whic...
Embodiment 3
[0037] Example 3 Preparation of [1-azabicyclo (2.2.2) octane-3S-yl]-(1,2,3,4-tetrahydronaphthalene-1S-methyl)amine
[0038] ①Reaction formula:
[0039]
[0040] ②Operation method:
[0041] In a 100ml three-necked flask, add 5g (17.6mmol) of compound and 100ml of tetrahydrofuran, add 2.8g (70.4mmol) of sodium borohydride in one go, add 12ml of boron trifluoride ether solution dropwise at room temperature, and then The reaction was stirred for 1 hour, then heated to reflux, and the reaction was continued for 2 hours. Cool to room temperature, add 6N hydrochloric acid under ice bath, adjust pH to about 1, and then reflux for 2 hours. Cool to room temperature, evaporate THF under reduced pressure, add 50% sodium hydroxide solution to adjust pH to alkaline, extract twice with chloroform, wash twice with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and recover solvent under reduced pressure , to obtain crude compound 4.70g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com