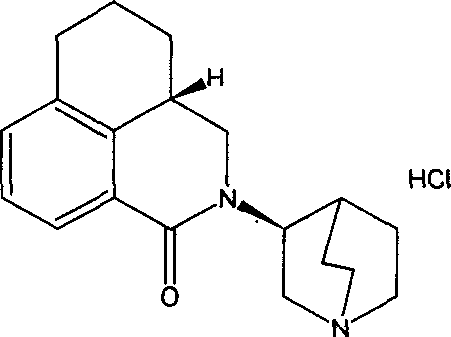
Palonosetron lyophilized formulation and its preparation method
A technology for palonosetron and freeze-dried preparations is applied in the field of freeze-dried preparations of palonosetron hydrochloride and its preparation, which can solve the problems such as no literature reports of palonosetron powder injection, and achieve the storage of medicines. The effect of extended period, good stability and product quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve the prescribed amount of glucose in an appropriate amount of water for injection, add an appropriate amount of activated carbon for needles, heat and stir, filter and decarbonize, and add accurately weighed 0.25 g of palonosetron hydrochloride (calculated in bases) to the fine filtrate cooled to room temperature , dissolve, adjust the pH to 3.70-3.99 with 0.1M HCL, add water to the prepared volume of 1000ml, that is, the concentration of palonosetron is 0.25mg / ml, filter and sterilize with a 0.22u mixed cellulose ester microporous membrane, press each bottle 1ml is divided into vials, freeze-dried to powder injection, corked under vacuum, sealed with an aluminum cap, and labeled.
[0029] The advantage of the present invention is that palonosetron hydrochloride adopts the freeze-dried preparation form and has better stability than the aqueous injection form. The palonosetron hydrochloride powder injection of the present invention is investigated for stability. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com