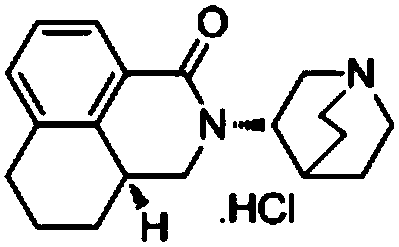
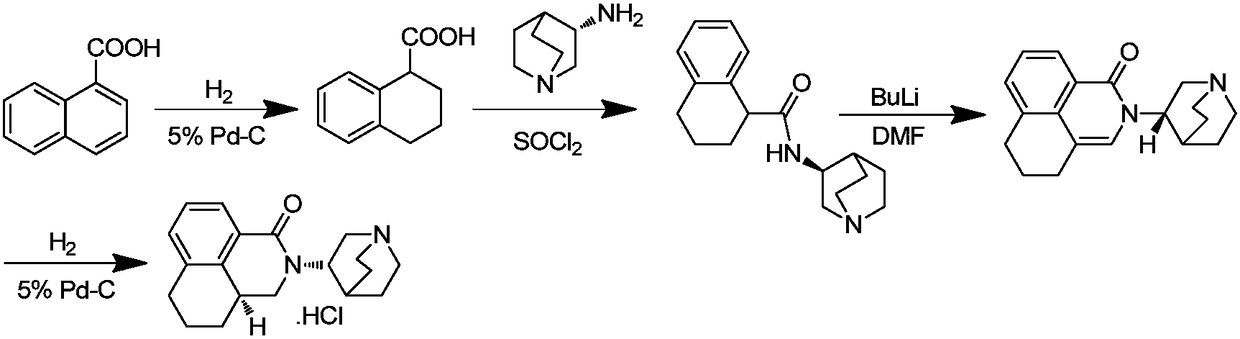
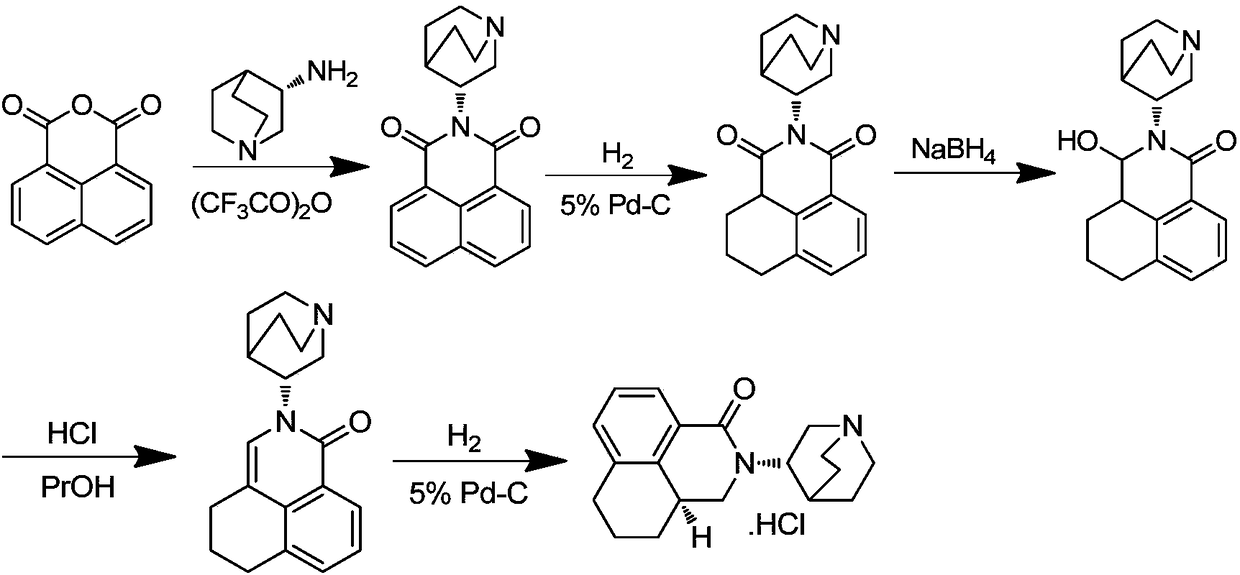
Preparation method of high-purity palonosetron hydrochloride
A palonosetron and high-purity technology, applied in the field of preparation of high-purity palonosetron hydrochloride, can solve the problems of low purity, low yield, unstable process and the like of palonosetron hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] A: Synthesis of (S)-1,2,3,4-tetralincarboxylic acid
[0093] Add 1,2,3,4-tetrahydronaphthoic acid (17.6g, 0.1mol) and quinine (32.4g, 0.1mol) into 176ml of 50% ethanol, stir at 50°C for 30min, the solution dissolves, and TLC detects the reaction Complete (PE:EA=1:2). Cool down to below 0°C and place overnight, crystallize, filter with suction, and dry. Add 100ml of ethanol to refine, crystallize below 0°C, filter with suction, dry, add 60ml of 1mol / L hydrochloric acid and 120ml of ethyl acetate, separate, dry, filter, concentrate, add n-hexane to the residue for recrystallization, and obtain a white solid 7.50 g, the yield is 42.6%, and the purity shown by HPLC is 99.81%, [α] D =-61.2° (c=1.0325, toluene).
[0094] B: Synthesis of (R)-N-((S)-3-quinyl)-1,2,3,4-tetrahydronaphthyl-1-carboxamide
[0095] Dissolve compound (S)-1,2,3,4-tetrahydronaphthoic acid (17.6g, 0.1mol) in 50ml of ethyl acetate, add oxalyl chloride (10ml, 0.12mol) under stirring, stir at room temperat...
Embodiment 2
[0103] A. Synthesis of (S)-1,2,3,4-tetrahydronaphthoic acid:
[0104] 15.6 grams, 0.1mol of 1,2,3,4-tetrahydronaphthoic acid and 29.8 grams, 0.1mol of quinine were added to 158 milliliters of 50V / V% ethanol, stirred at 50°C for 30 minutes to dissolve the solution, and detected by TLC The reaction is complete, PE:EA=1:2, lower the temperature to below 0°C and place overnight, crystallize, filter with suction, dry, then add 90 ml of ethanol to refine, crystallize below 0°C, filter with suction, dry, add 55 ml 1mol / L hydrochloric acid and 100ml ethyl acetate, separated, dried, filtered, concentrated, and the residue was recrystallized by adding n-hexane to obtain 7.21g of (S)-1,2,3,4-tetrahydronaphthoic acid as a white solid , the yield was 43.6%, and the HPLC showed that the purity was 99.82%, [α] D =-61.0° (c=1.0333, toluene)
[0105] B. Synthesis of (R)-N-((S)-3-quinyl)-1,2,3,4-tetrahydronaphthyl-1-carboxamide
[0106] Dissolve 15.6 grams, 0.1mol of compound (S)-1,2,3,4-tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com