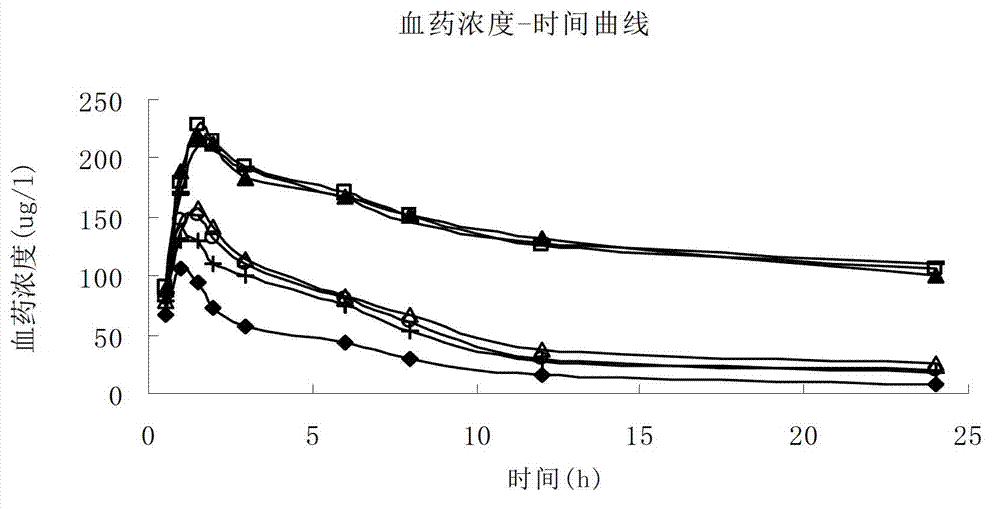
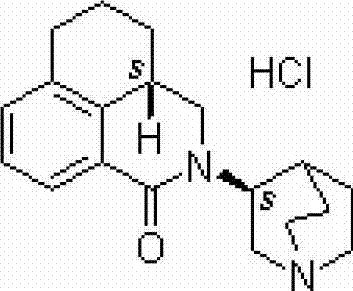
Palonosetron hydrochloride lipidosome injection
A technology of injection and agar, which is applied in the field of pharmaceutical preparations, can solve the problems of low stability of ordinary injection preparations, and achieve the effects of high quality, uniform particle size distribution and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] According to another aspect of the present invention, the preparation method of above-mentioned palonosetron hydrochloride liposome injection is provided, the method comprises the following steps:
[0063] (1) Dissolve cholesterol, soybean phosphatidylserine, phosphatidylglycerol, soybean sterol and Tween 80 in buffered saline solution to make blank liposomes;
[0064] (2) The blank liposomes prepared above were sterilized by flowing steam, and then sonicated twice for 20 minutes each time;
[0065] (3) Under sterile conditions, add palonosetron hydrochloride to the liposome in molten state, and add trehalose under constant stirring;
[0066] (4) Quickly freeze, then return to room temperature, constant volume, stir evenly, filter through a 0.22 μm microporous membrane, fill, and obtain palonosetron hydrochloride liposome injection.
[0067] In the method according to the present invention, in step (1), the buffered saline solution is selected from one of phosphate buf...
Embodiment 1
[0074] Example 1 Preparation of Palonosetron Hydrochloride Liposomal Injection
[0075] The raw materials used are as follows:
[0076]
[0077] The preparation process is as follows:
[0078] (1) Dissolve 6.5g of soybean phosphatidylserine, 2.5g of phosphatidylglycerol, 3.25g of soybean sterol and 1.5g of Tween 80 in 500ml of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH of 7.0 to make a blank Liposomes;
[0079] (2) The blank liposomes prepared above were sterilized by flowing steam, and then sonicated twice for 20 minutes each time;
[0080] (3) Under sterile conditions, add 0.25 g of palonosetron hydrochloride to the liposome in molten state, and add 3.25 g of trehalose under constant stirring;
[0081] (4) Quickly freeze at -40°C, then return to room temperature, add water for injection to a volume of 5000ml, stir evenly, filter with a 0.22μm microporous membrane, fill (5ml / bottle), and obtain 1000 bottles of palonosetron hydrochl...
Embodiment 2
[0082] Example 2 Preparation of Palonosetron Hydrochloride Liposomal Injection
[0083] The raw materials used are as follows:
[0084]
[0085] The preparation process is as follows:
[0086] (1) Dissolve 5g of soybean phosphatidylserine, 2.5g of phosphatidylglycerol, 2.5g of soybean sterol and 1.25g of Tween 80 in 500ml of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH of 7.0 to make a blank fat plastid;
[0087] (2) The blank liposomes prepared above were sterilized by flowing steam, and then sonicated twice for 20 minutes each time;
[0088] (3) Under sterile conditions, add 0.25 g of palonosetron hydrochloride to the liposome in molten state, and add 2.5 g of trehalose under constant stirring;
[0089] (4) Quickly freeze at -40°C, then return to room temperature, add water for injection to a volume of 5000ml, stir evenly, filter with a 0.22μm microporous membrane, fill (5ml / bottle), and obtain 1000 bottles of palonosetron hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com