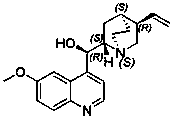
Recovering and reusing method of resolving agent quinine
A kind of resolving agent, separation technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] recycling part
[0020] The crystallization mother liquor in the above reaction was placed in a 60°C water bath and concentrated under reduced pressure to about 120ml, then added 130ml of 1mol / L hydrochloric acid aqueous solution, and then 150ml of dichloromethane, stirred for 1 hour, and stood to separate layers. Add 80ml of 2mol / L sodium hydroxide aqueous solution to the upper aqueous phase, and measure pH=13.12 with a Mettler pH meter; add 250ml of dichloromethane, stir for 1 hour, and let stand to separate layers; collect the dichloromethane phase, and then use 200ml of dichloromethane Extract with methane, combine the dichloromethane phases, add 30 g of anhydrous sodium sulfate to dry, then place in a water bath at 40°C and concentrate to dryness. Add 200ml of ethanol to the concentrate, raise the temperature to reflux and stir for 1h, then cool down to -10°C and stir for 4h. After filtration, the filter cake was air-dried in an oven at 60° C. to dryness to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com