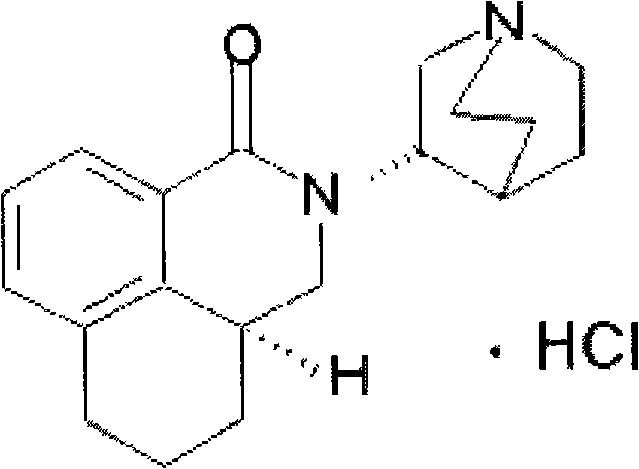
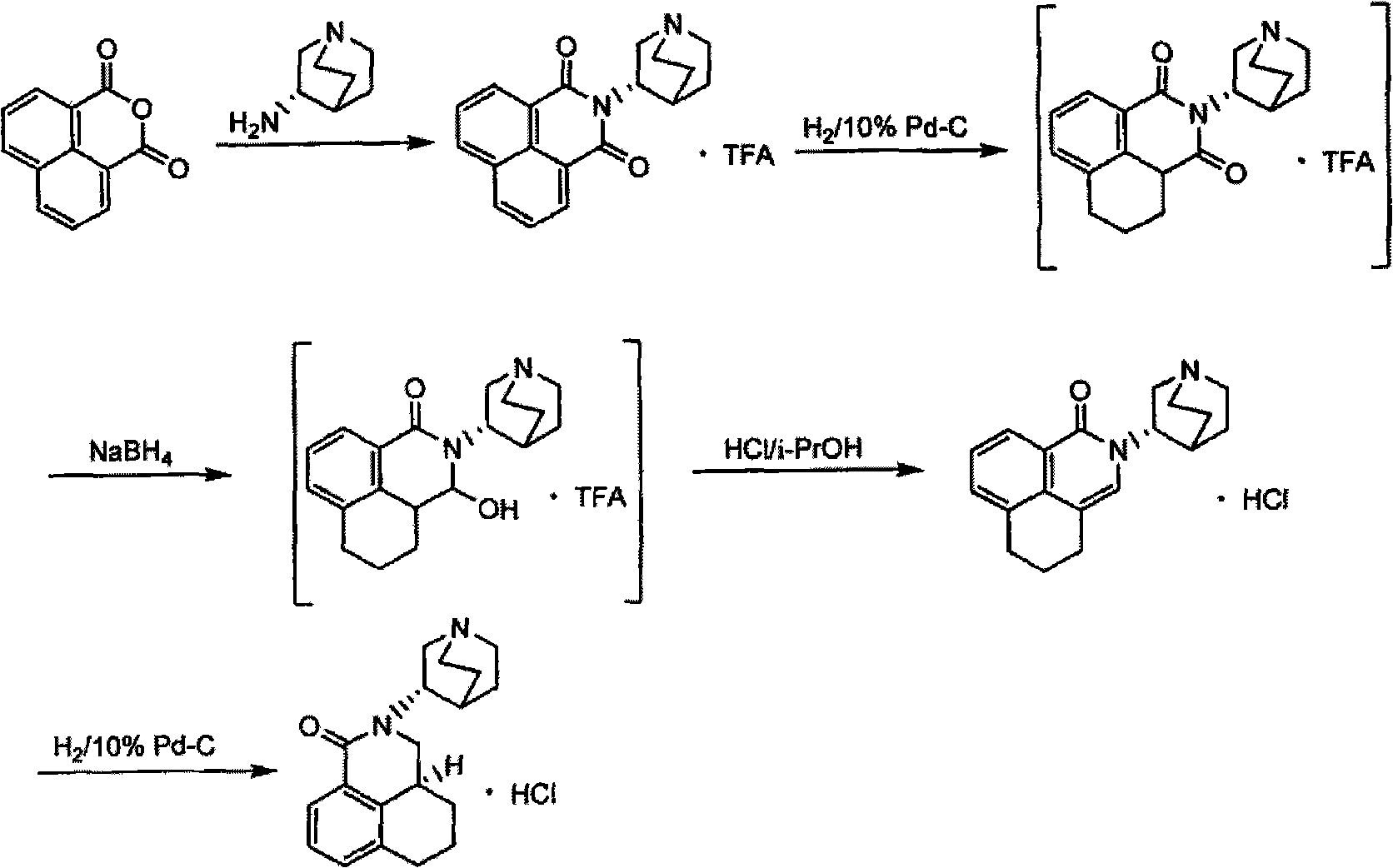
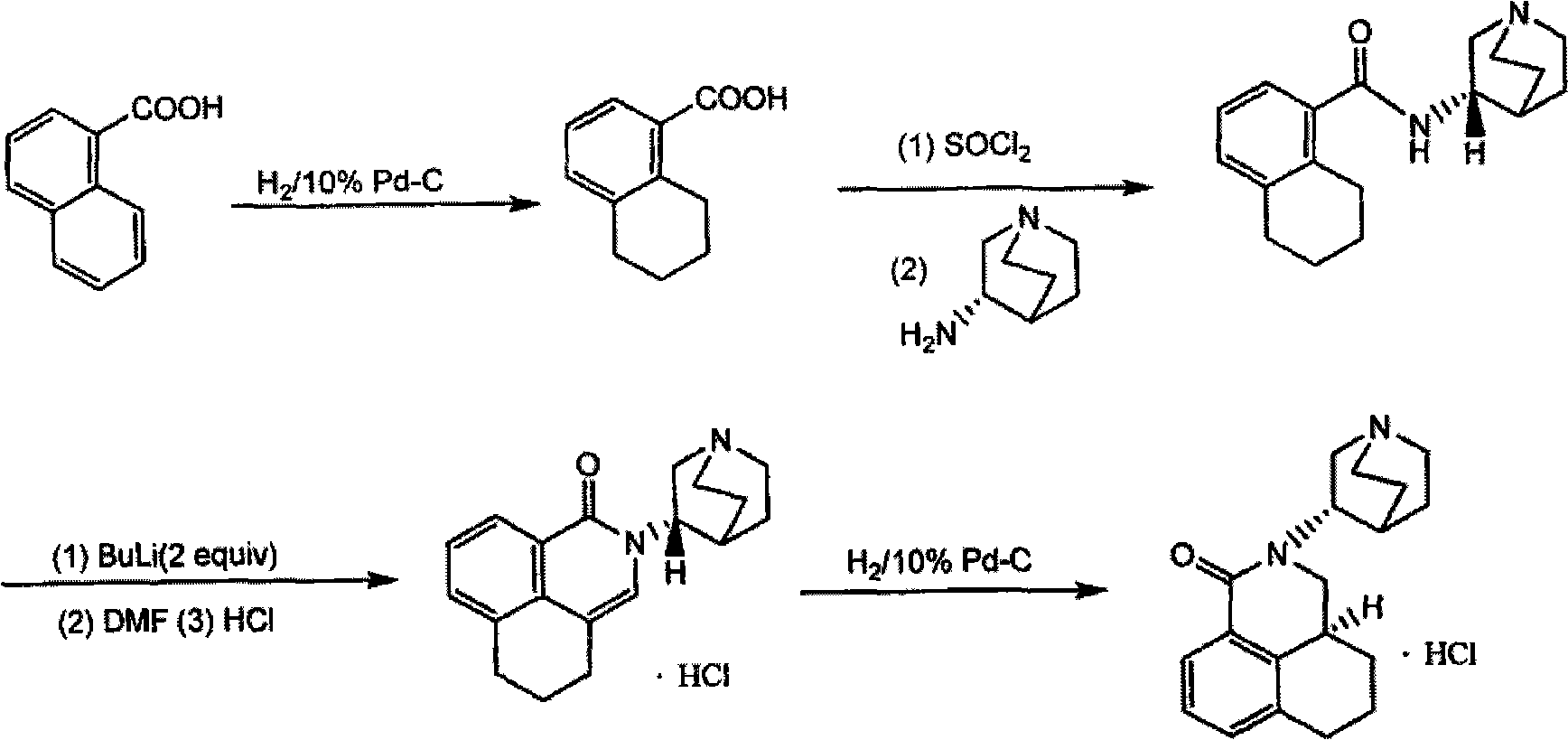
Preparation method of palonosetron and palonosetron hydrochloride and injection
A technique for palonosetron and jone injections, applied in the field of injections, preparation of palonosetron hydrochloride, and palonosetron, can solve problems such as difficult product quality, high process requirements, and low product purity, and achieve Low cost, simple process and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Step 1, the synthesis of N-(1-aza-3(S)-bicyclo[2,2,2]octyl)-1,2,3,4-tetrahydronaphthalene-1S-carboxamide
[0051]
[0052] Put 300 g of 1,2,3,4-tetrahydro-1S-naphthoic acid into a 5 L reaction flask, add 3 L of freshly distilled toluene, stir until completely dissolved and the reaction solution is clear. Under ice-cooling conditions, 20ml of N,N-dimethylformamide was added dropwise. Keeping the temperature of the reaction system below 20°C, 0.56 L of thionyl chloride was slowly added dropwise, and the reaction was stirred at room temperature for 1 h after the dropwise addition was completed. Stirring was then continued at 50°C, and the reaction progress was tracked by TLC (developing solvent: petroleum ether: ethyl acetate = 3:1), and the reaction was complete after about 3 hours. Concentrate under reduced pressure to remove the solvent, add 0.5 L of freshly distilled toluene and continue to concentrate under reduced pressure until no solvent flows out, and add 0.5 ...
Embodiment 2
[0131] The preparation of embodiment 2 palonosetron hydrochloride injection
[0132] The raw material is: palonosetron hydrochloride 280mg (in C 19 h 24 N 2 O is 250mg), the auxiliary materials are mannitol 207.5g, sodium citrate 9.32g, citric acid 3.85g and edetate calcium sodium 2.5g, water for injection is added to 5000ml, made 1000, specification: 5ml: 0.25 mg (in C 19 h 24 N 2 O meter).
[0133] Weigh the sodium citrate, citric acid, mannitol and calcium sodium edetate of the above weight, add 2000ml of water for injection, stir and dissolve, then add medicinal activated carbon of 0.05% (W / V) of the total amount of the solution, and heat at 60°C Insulate and absorb for 15 minutes, filter and set aside.
[0134] Take by weighing the above-mentioned palonosetron hydrochloride in the water for injection of 1000ml, dissolve completely, set aside.
[0135] After mixing the above two solutions, add water for injection to make up to 5000ml, adjust the pH value to 4.5-5.5...
Embodiment 3
[0137] The pilot test of embodiment 3 palonosetron hydrochloride injection
[0138] Prepare three batches of pilot product according to the prescription of Table 7, each 10000, the yield is shown in Table 7.
[0139] Table 7 Proportion and yield of three batches of pilot test samples
[0140]
[0141] Table 8 lists the test results of the pilot test samples:
[0142] Table 8 Test results of three batches of samples
[0143]
[0144] It can be seen from the above table that the three batches (06040301, 06040501, 06040701) of samples prepared by the pilot test all meet the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com