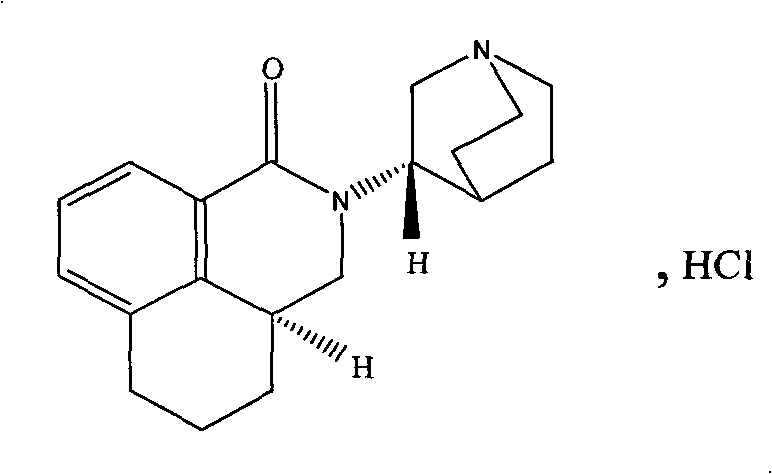
Method for simultaneously determining four optical isomers of palonosetron hydrochloride
A technology of optical isomers and palonosetron, which is applied in the fields of organic chemistry, chemical instruments and methods, organic chemistry, etc., can solve the analytical method that has not yet been reported to achieve chromatographic separation at one time, does not achieve one-time separation, and is cumbersome. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
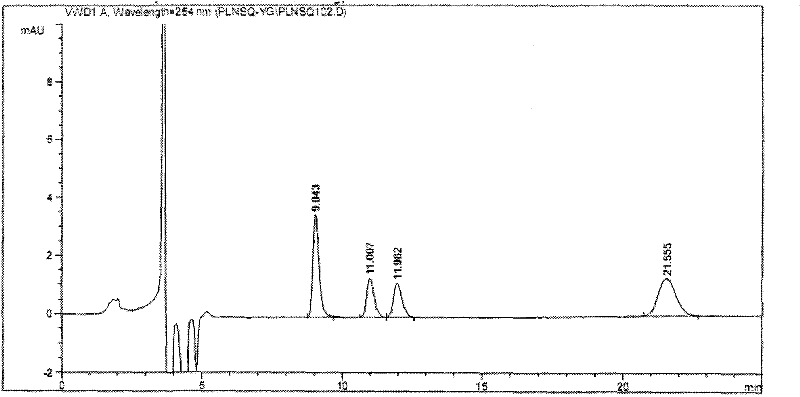
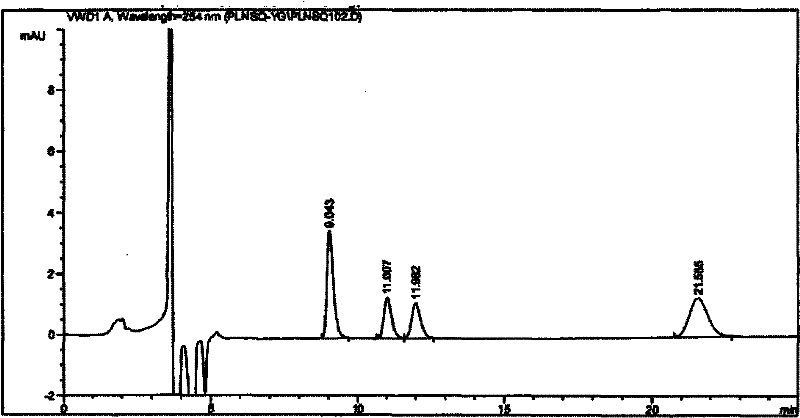
[0017] Embodiment, simultaneous determination of four optical isomers of palonosetron hydrochloride
[0018] 1. Instruments and reagents
[0019] 1.1 Instruments and reagents
[0020] Instrument: Agilent 1100series high performance liquid chromatography, chromatographic grade acetonitrile, isopropanol (Merck), diethylamine (analytical grade).
[0021] Reagents: S.R, R.S, R.R type isomers (self-made or purchased from the market), palonosetron hydrochloride raw materials (provided by Shenzhen Neptune Pharmaceutical Co., Ltd., batch numbers 20080601, 20080602, 20080603).
[0022] 1.2 Chromatographic conditions:
[0023] Chromatographic column: CHIRALPAK OD-H 5μm 4.6×250mm chiral chromatographic column (manufactured by DAICEL)
[0024] Mobile phase: acetonitrile: isopropanol: diethylamine (the volume ratio of the three is 82-92: 18-8: 0.1-0.2; and preferably 85: 15: 0.1)
[0025] Flow rate: 0.3-1.2ml / min (preferably 1.0ml / min), injection volume: 5μl.
[0026] Column temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com