Analysis method of impurity C, impurity D and enantiomer in palonosetron hydrochloride injection or bulk drug
A technology for palonosetron and enantiomers, which is applied in the field of analytical chemistry, can solve the problems of unsuitable detection of impurities C, D and enantiomers, and achieves the effect of reducing time and reagent costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
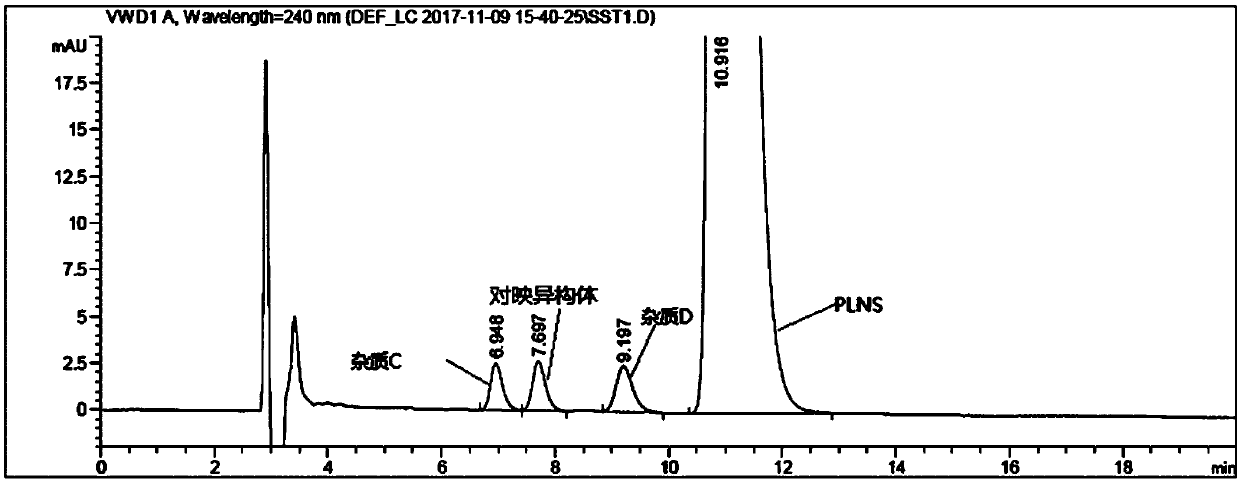
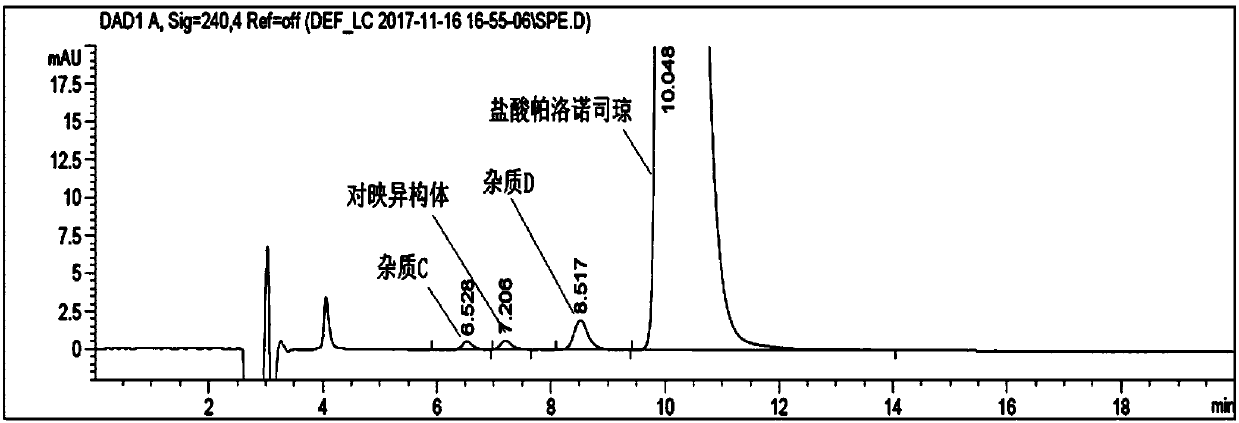
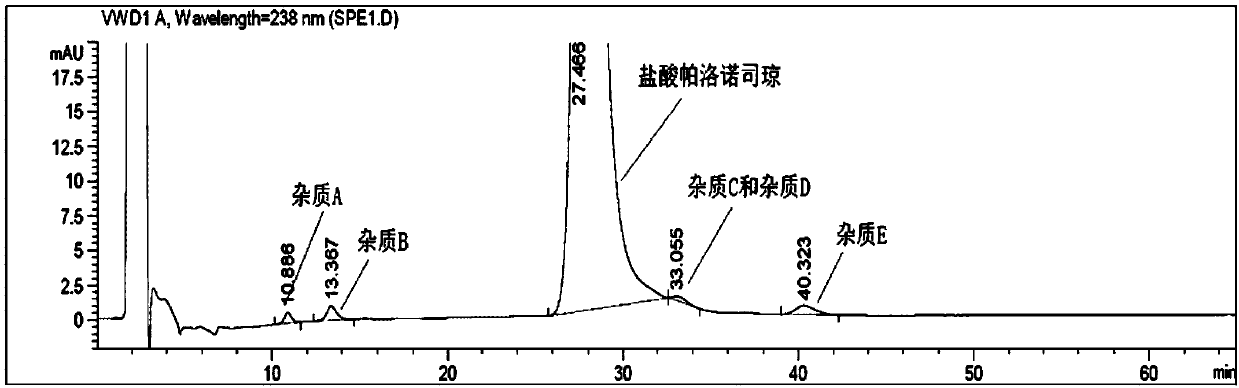
[0022] Take 5.0ml of palonosetron hydrochloride injection, put it in a 20ml test tube, add 2.0ml of 10mol / L sodium hydroxide solution, and shake for 1min to free the palonosetron hydrochloride. Then add 1.0ml of n-hexane, shake it, let it stand until the layers are separated (about 5min), and take the upper n-hexane phase as the test solution. Take an appropriate amount of impurity C, impurity D and enantiomer reference substance, add ethanol to dissolve and make a solution containing about 0.06mg per 1ml, as impurity stock solution. Take another appropriate amount of palonosetron hydrochloride reference substance and impurity stock solution, and dilute with mobile phase to make a solution containing about 0.24 mg of palonosetron and 1.2 μg of each isomer per 1 ml, as a system suitability solution. Measure according to high performance liquid chromatography (general rules 0512), use polysaccharide-coated chiral column, n-hexane-methanol-diethylamine (70:30:0.1) is mobile phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com