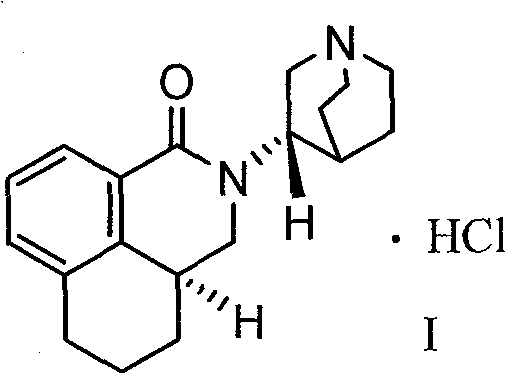
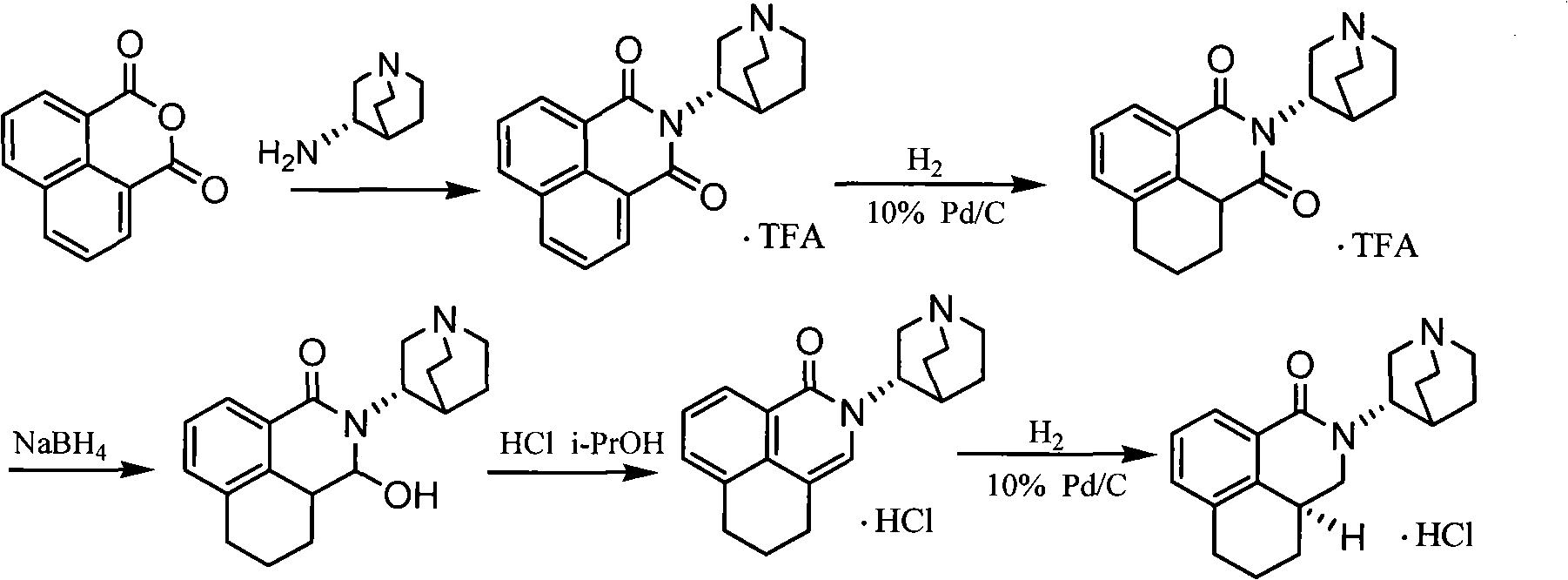
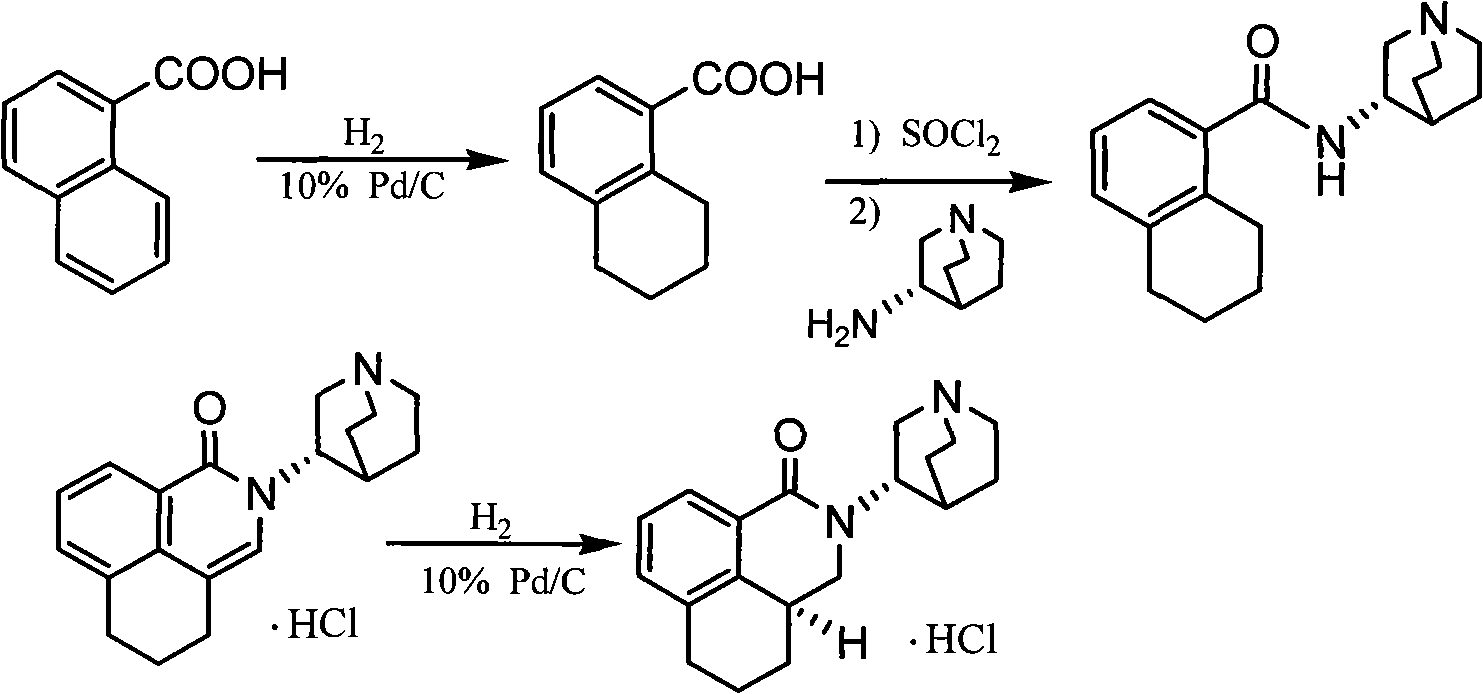
Palonosetron hydrochloride, and precursor compound and preparation method thereof
A technology of precursor compounds and compounds, applied in the direction of digestive system, organic chemistry, drug combination, etc., can solve the problems of safety and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 8-chloromethyl-1-naphthoic acid compound
[0030] Mix 1-naphthoic acid (4.3kg, 25mol), paraformaldehyde (1.5kg, 50mol), acetic acid (3.25L) and concentrated hydrochloric acid (4.5L), heat to 80-85°C, and stir vigorously for 6h. After the reaction is complete, cool to room temperature, adjust the pH to 3 with sodium bicarbonate under an ice-water bath. Filter with suction, wash with water (500ml×2), and dry the obtained solid at 80°C for 10h. The crude product was recrystallized from toluene to give 8-chloromethyl-1-naphthoic acid (3.5 kg, m.p. 177-178°C). ESI-MS (positive mode) m / z: 221.4[M+1]+, 1HNMR (400MHz, CDCl3): δ8.36 (1H, d, J=7.2Hz), 8.10 (1H, d, J=8.4Hz ), 7.86 (1H, d, J = 8.4Hz), 7.63 (1H, t, J = 7.6, 8.0Hz), 7.57 (1H, t, J = 7.6, 8.0Hz), 7.38 (1H, d, J = 6.8Hz), 4.95 (2H, s).
Embodiment 2
[0031] Example 2 Preparation of 8-[(3S)-1-azabicyclo[2.2.2]octyl]-aminomethyl-1-naphthoic acid
[0032] The product 8-chloromethyl-1-naphthoic acid (3.3kg, 15mol) of embodiment 1, S-3-aminoquinuclidinine (1.89kg, 15mol) and potassium carbonate (4.1kg, 30mol) are placed in ethanol , Water mixed solvent (1 / 1 ethanol / water, 50L), reflux for 5h. Cool to room temperature, add 2L of concentrated hydrochloric acid, stir at room temperature for 2h, spin dry the solvent under reduced pressure, add 50L of ethanol, stir at room temperature for 2h, filter to remove insoluble matter. The solvent was spin-dried under reduced pressure to obtain 8-[(3S)-1-azabicyclo[2.2.2]octyl]-aminomethyl-1-naphthoic acid (4.5kg, m.p.>270°C (dec.), (c=1, CHCl3), ESI-MS (positive mode) m / z: 311.3[M+1]+, 1HNMR (400MHz, CDCl3): δ8.27 (1H, d, J=7.2Hz), 7.91 ( 1H, d, J = 8.4Hz), 7.70 (1H, d, J = 8.4Hz), 7.53 (1H, t, J = 7.6, 8.0Hz), 7.33 (1H, t, J = 7.6, 8.0Hz), 7.14(1H, d, J=6.8Hz), 4.47-4.49(m, 1H), 3.99-4...
Embodiment 3
[0033] Example 3 Preparation of 2-[(3S)-1-azabicyclo[2.2.2]octyl]-2,3-dihydro-1H-benzo[de]isoquinolin-1-one
[0034] The product 8-[(3S)-1-azabicyclo[2.2.2]octyl]-aminomethyl-1-naphthoic acid (3.1kg, 10mol), DCC (2.06kg, 10mol) of embodiment 2 Stir in 50 L of dichloromethane at room temperature for 5 h, filter and concentrate the reaction mixture to give 2-[(3S)-1-azabicyclo[2.2.2]octyl]-2,3-dihydro-1H-benzene And[de]isoquinolin-1-one (2.89kg, m.p.148-149°C, (c=1, CHCl3), ESI-MS (positive mode) m / z: 293.1[M+1]+, 1HNMR (400MHz, CDCl3): δ8.31 (1H, d, J=7.2Hz), 8.02 (1H, d, J = 8.4Hz), 7.79 (1H, d, J = 8.4Hz), 7.63 (1H, t, J = 7.6, 8.0Hz), 7.49 (1H, t, J = 7.6, 8.0Hz) , 7.31(1H, d, J=6.8Hz), 4.45-4.71(m, 3H), 3.91(m, 1H), 3.79(m, 2H), 3.38(m, 3H), 2.49(m, 1H), 1.89-2.34 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com