Method for separating and measuring Palonosetron hydrochloride and optical isomers thereof
A technology of optical isomers and palonosetron, applied in the field of separation and determination of palonosetron hydrochloride and its optical isomers, can solve the problems of chromatographic peaks that cannot be separated, separated, separated, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Chromatography, mass spectrometry and detection limit basic research of four kinds of optical isomers of embodiment 1
[0027] Experimental equipment and conditions
[0028] a, HPLC (high performance liquid chromatography) conditions
[0029] Instrument: SHIMADZU LC-2010A HT (Shimadzu, Japan)
[0030] Detector: UV
[0031] Mobile phase: 0.3% triethylamine (adjust pH4.0 with glacial acetic acid)-methanol (30:70)
[0032] Chromatographic column: CHIROBIOTIC T 4.6×250mm 5μm
[0033] Flow rate: 1.0ml / min
[0034] Detection wavelength: 249nm
[0035] Chromatography Workstation: LCsolution
[0036] b. MS (mass spectrometry) conditions
[0037] MS instrument: API3000 (American Bio-Application Engineering Company)
[0038] Ion Source: Turbo Spray
[0039] Ion Source Gas1 (GS1): 4.0
[0040] Curtain gas: 10.0
[0041] Ion Source Gas2 (GS2): 0.0
[0042] DP: 60.0
[0043] EP: 12.0
[0044] IS: 5500.00
[0045] method study
[0046] a. LC qualitative
[0047] Tak...
Embodiment 2
[0061] Determination of other optical isomers content in the Palonosetron hydrochloride bulk drug in embodiment 2
[0062] a, Determination method
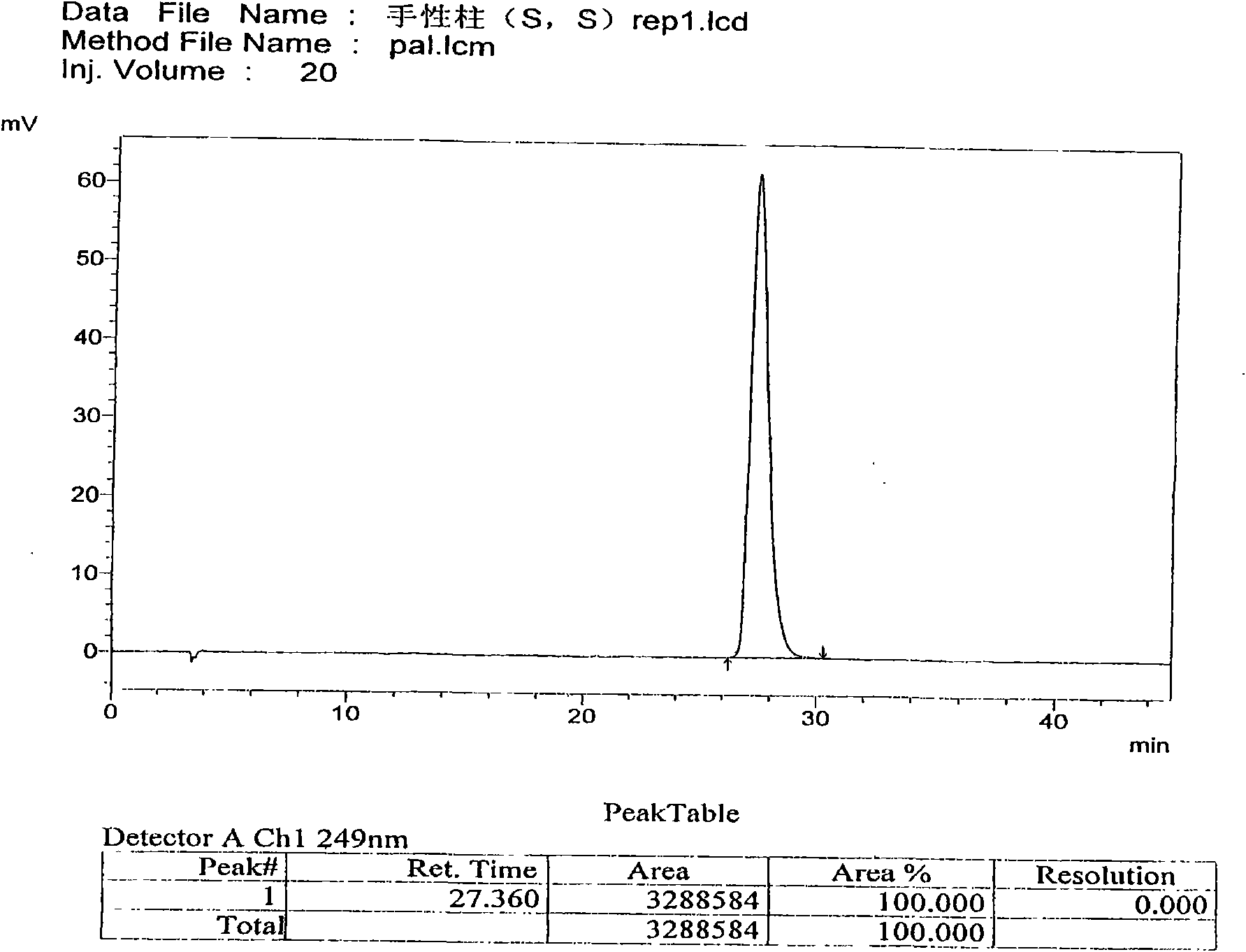
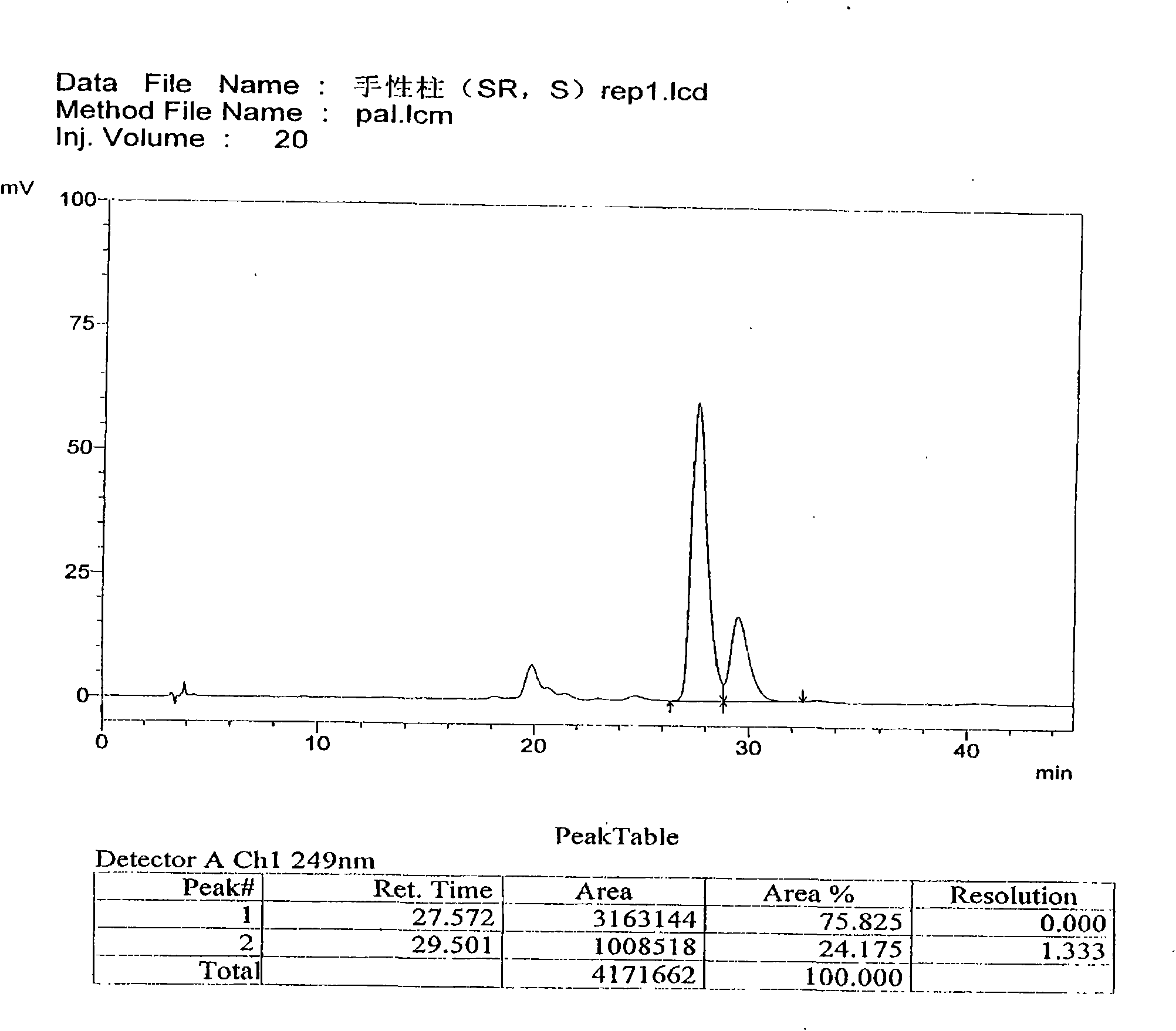
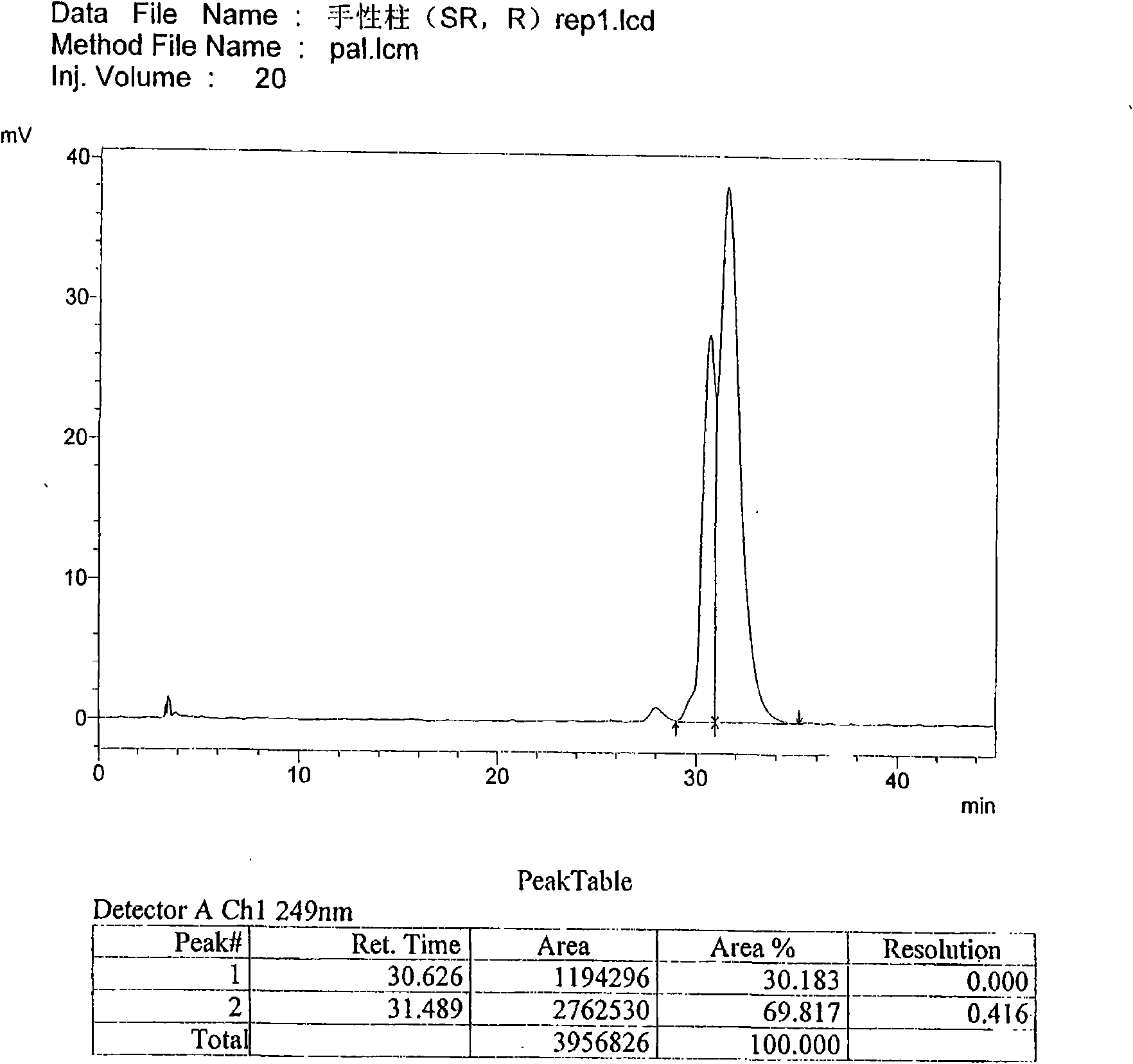
[0063] Use macrocyclic glycopeptide Teicoplanin (Teicoplanin) bonded silica gel as filler (CHIROBIOTIC T 4.6×250mm5μm); use 0.3% triethylamine (adjust pH4.0 with glacial acetic acid)-methanol (30:70) as mobile phase ; The detection wavelength is 249nm. Take an appropriate amount of (3aS, S), (3aR, S), (3aS, R), (3aR, R) mixtures, add mobile phase to dissolve and dilute to a solution of about 0.2mg per 1ml, and inject samples according to the above chromatographic conditions 20 μl, record the chromatogram. According to the order of peaks, peak 1 is (3aS, S), peak 2 is (3aR, S), peak 3 and peak 4 are (3aS, R), two components of (3aR, R) mixture. The number of theoretical plates calculated by (3aS, S) should not be less than 4000, and the separation degree of palonosetron hydrochloride peaks and adjacent isomers should not be less...
Embodiment 3
[0071] Other optical isomer content determination in embodiment 3 Palonosetron hydrochloride injection
[0072] a, Determination method
[0073] Use macrocyclic glycopeptide Teicoplanin (Teicoplanin) bonded silica gel as filler (CHIROBIOTIC T4.6×250mm5μm); use 0.3% triethylamine (adjust pH4.0 with glacial acetic acid)-methanol (30:70) as flow phase; the detection wavelength is 249nm. Take an appropriate amount of (3aS, S), (3aR, S), (3aS, R), (3aR, R) mixtures, add mobile phase to dissolve and dilute to a solution of about 0.2mg per 1ml, and inject samples according to the above chromatographic conditions 20 μl, record the chromatogram. According to the order of peaks, peak 1 is (3aS, S), peak 2 is (3aR, S), peak 3 and peak 4 are (3aS, R), two components of (3aR, R) mixture. The number of theoretical plates calculated by (3aS, S) should not be less than 4000, and the separation degree of palonosetron hydrochloride peaks and adjacent isomers should not be less than 1.0
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com