Compositions for treating centrally mediated nausea and vomiting
A composition and pharmaceutical dosage form technology, applied in the field of combined oral dosage forms of palonosetron and netupitant, can solve the problem of not specifically mentioning nausea and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: the preparation of oral dosage form
[0102]In a preferred embodiment, the combination is administered in the oral dosage form of a capsule, wherein the capsule encapsulates one or more soft gel capsules of palonosetron and one or more hard tablets of netupitant . Table 1 below describes a representative formulation of a soft gel capsule containing 0.5 mg of palonosetron suitable for inclusion in such a hard shell.
[0103] Table 1: Representative Soft-Gel Formulations
[0104]
[0105]
[0106] 1 Equivalent to 0.50mg free base
[0107] 2 The quantitative composition of the capsule shell is proprietary to Catalent Pharma Solutions
[0108] Table 2 below describes a representative formulation of a tablet containing 100 mg of netupitant suitable for inclusion in a hard shell.
[0109] Table 2: Representative Tablet Formulations
[0110] components Approximate amount (mg / tablet) Features Netupitant, Triturated 100 active age...
Embodiment 2
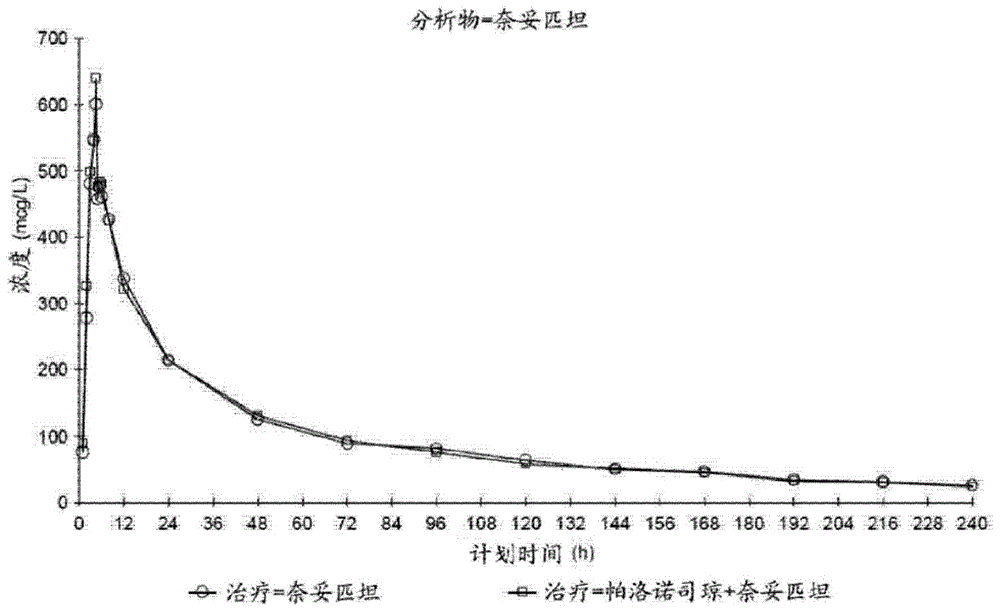
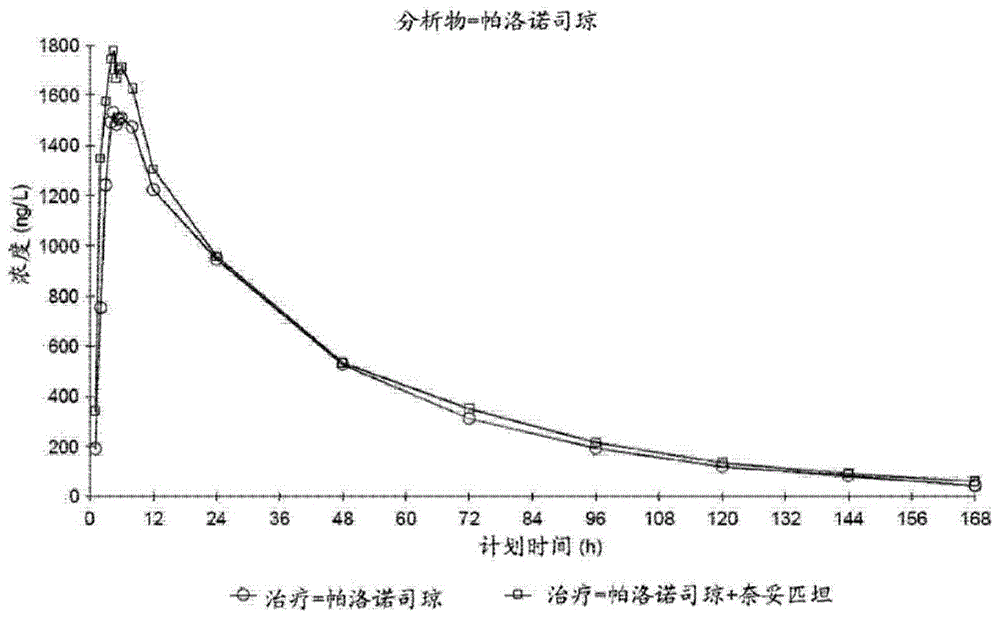
[0111] Example 2: Pharmacokinetics of Combination Dosage Forms
[0112] Purpose
[0113] The effect of palonosetron on the pharmacokinetics (PK) of netupitant and the effect of netupitant on the PK of palonosetron were examined in healthy volunteers.
[0114] method
[0115] A randomized, open three-way crossover study was performed. Each subject participated in 3 treatment periods, each lasting approximately 12 days (Day -1 to Day 11). The treatment periods are separated by a rest period (between Day 1 of any two consecutive treatment periods) of not less than 14 days.
[0116] The following treatments were studied:
[0117] Treatment A: Netupitant 450 mg was given orally as a single dose in three 150 mg capsules.
[0118] Treatment B: Palonosetron 0.75 mg and Netupitant 450 mg were administered orally simultaneously as three 150 mg netupitant capsules followed by one 0.75 mg palonosetron capsule.
[0119] Treatment C: Palonosetron 0.75 mg was administered orally in a s...
Embodiment 3
[0131] Example 3: Netupitant + Dexamethasone Drug Interaction Study
[0132] In this study, the effect of netupitant on the pharmacokinetics of orally administered dexamethasone was evaluated. This was a randomized, open-label, three-period crossover study using an incomplete Latin Square design in which subjects were given dexamethasone alone, or netupitant 100 mg, 300 mg or 450 mg orally with dexamethasone. Netupitant was given orally on day 1 only. The dexamethasone regimen for each treatment was 20 mg orally on day 1 and 8 mg orally every 12 hours on days 2 to 4. Nineteen subjects (12 males, 7 females) completed the study (ie, all 3 treatment periods).
[0133] Mean plasma concentrations of dexamethasone were higher when dexamethasone was co-administered with netupitant ( Figure 4 ). This increase appears to be dependent on the presence of netupitant.
[0134] The AUC of dexamethasone when co-administered with 100, 300 and 450 mg of netupitant 0-24 (Day 1) is 1.5 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com