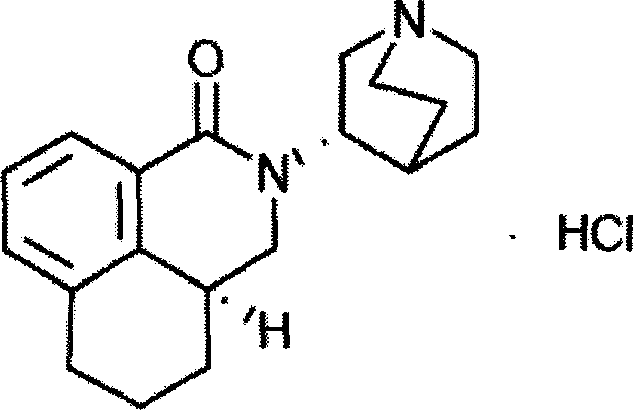
Palonosetron hydrochloride intravenous injection medicinal composition and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, digestive system, etc., can solve problems such as vascular irritation, decreased blood calcium concentration, and blood coagulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Palonosetron Hydrochloride Injection
[0024] Specifications: 5ml: 0.25mg (based on palonosetron C 19 h 24 N 2 O meter).
[0025] The prescription is as follows:
[0026] Palonosetron Hydrochloride
280mg (in C 19 h 24 N 2 O counted as 250mg)
207.5g
sodium citrate
9.41g
3.89g
2.5g
Add water for injection to
5000ml
production
1000 sticks (5ml:0.25mg, in C 19 h 24 N 2 O meter)
[0027] a. Weigh 207.5g of mannitol, 3.89g of citric acid, 9.41g of sodium citrate and 2.5g of calcium sodium edetate, add water for injection, stir to dissolve, then add medicinal activated carbon, stir, stand still, and filter Get solution I for subsequent use;
[0028] b. Weigh 280 mg of palonosetron hydrochloride, add it to water for injection, dissolve completely, and obtain solution II for later use;
[0029] c. After mixing solution I and solutio...
Embodiment 2
[0032] Take 56mg of palonosetron hydrochloride, 41.5g of mannitol, 798mg of citric acid and 1882mg of sodium citrate in proportional amounts, dissolve them in an appropriate amount of water for injection, add water for injection to 1000ml, take four portions, each 200ml, respectively Add 0.00%, 0.03%, 0.05%, 0.10% (w / v) calcium sodium edetate, stir to dissolve, and filter with 0.22 μm microporous membrane respectively. Investigate its appearance, pH value, content and related substances. The results are shown in Table 2.
[0033] Table 2 Antioxidant results of different amounts of edetate calcium sodium
Embodiment 3
[0036] Prepare about 0.01mol / L citric acid-sodium citrate buffer solution, 1000ml each of sodium acetate-acetic acid or sodium hydroxide-hydrochloric acid, add the prescribed amount of palonosetron hydrochloride and mannitol respectively, stir and dissolve, and adjust the pH value to about 5.0, filtered with a 0.22 μm microporous membrane, the appearance, content, and determination results of related substances are shown in Table 1.
[0037] Table 3 Investigation results of each test item when pH=5.0
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com