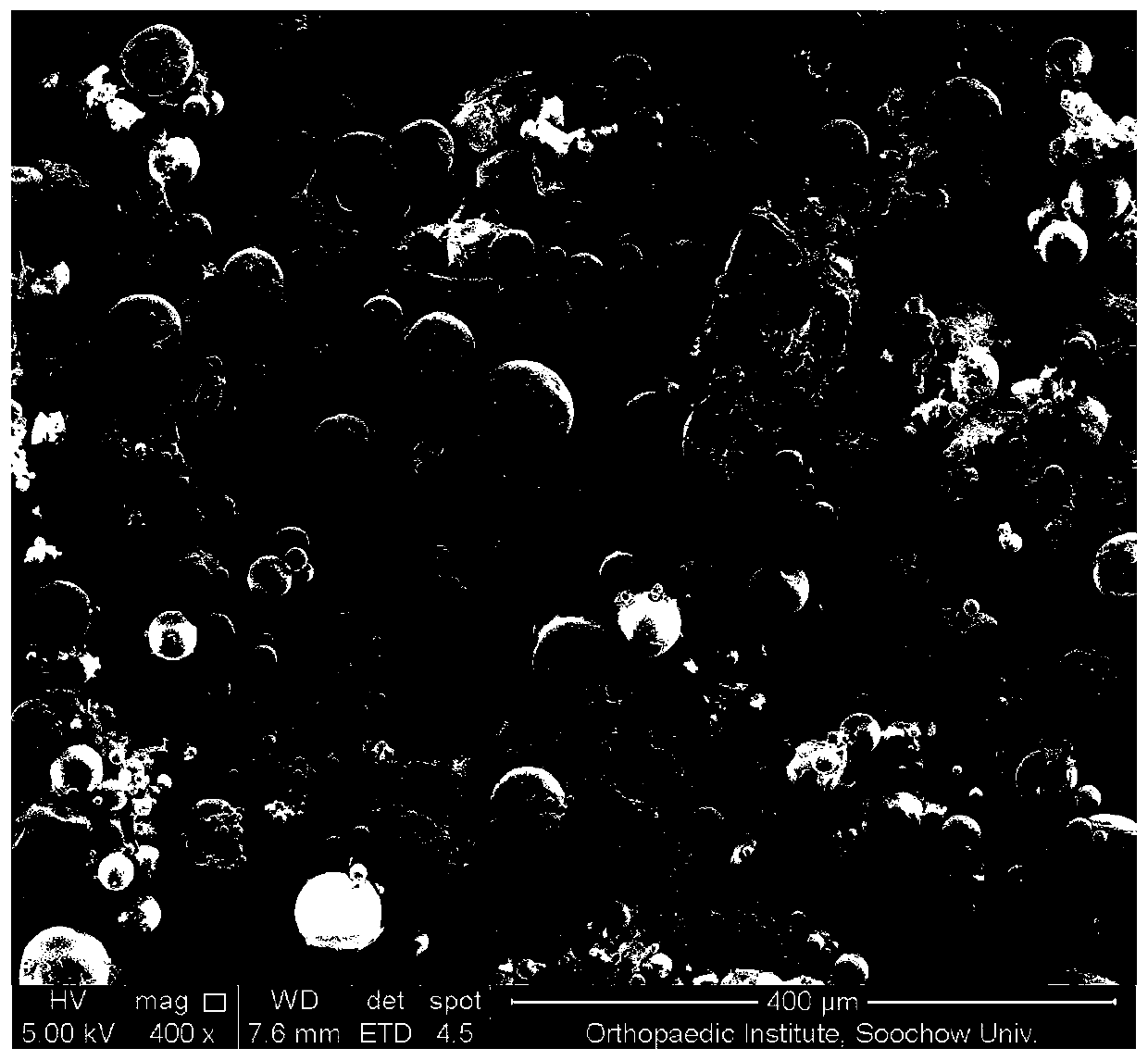
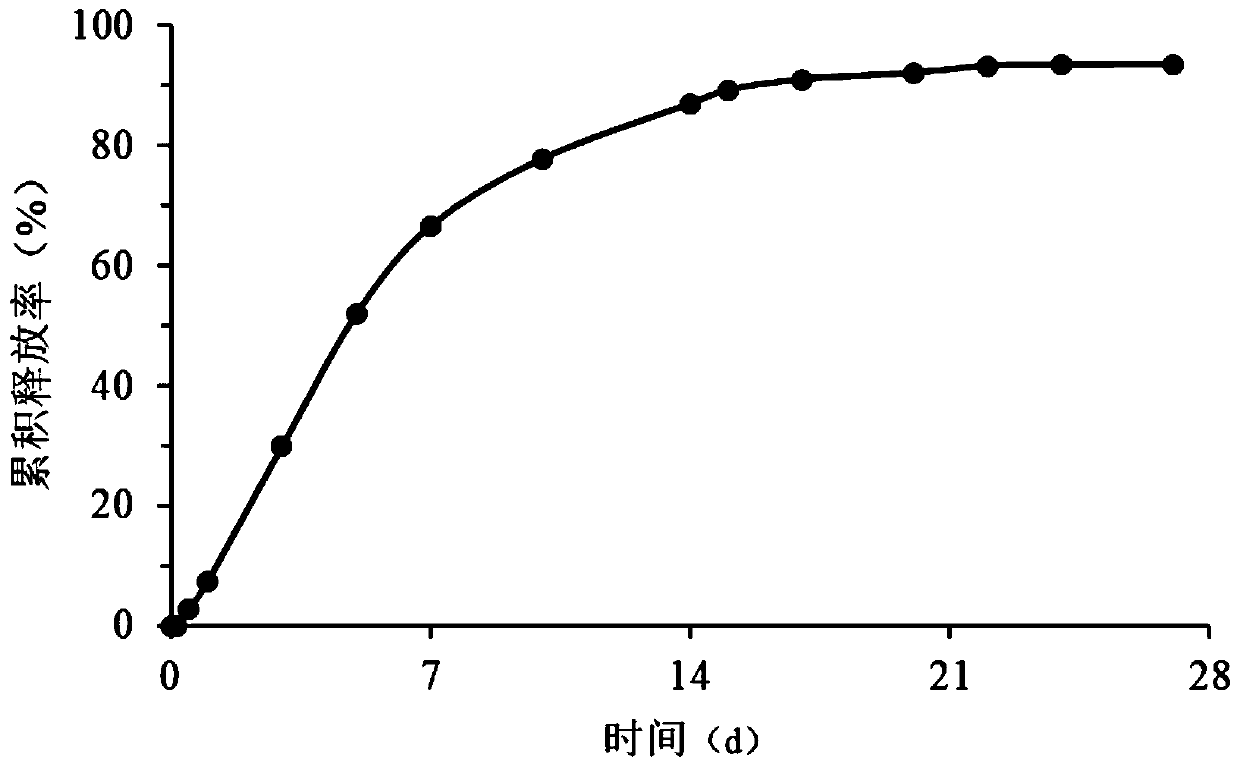
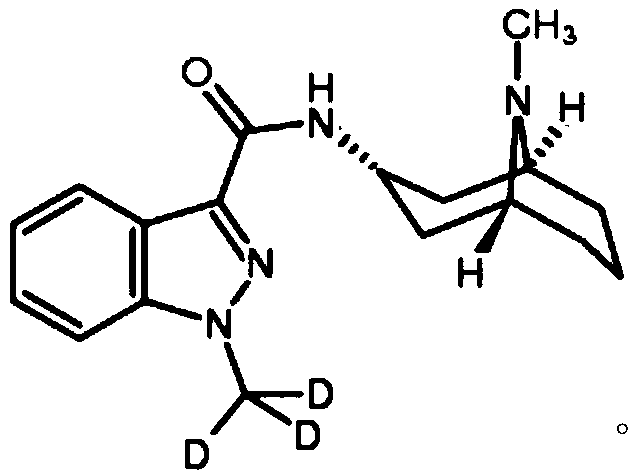
Granisetron sustained-release microspheres and preparation method thereof
A technology of sustained-release microspheres and hydrophobic segments, which is used in pharmaceutical formulations, medical formulations with inactive ingredients, and medical formulations containing active ingredients, etc., can solve the problems of poor patient compliance and short formulation release time, and achieve The effect of slow release of drug load, reduced drug loss and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 5 mg of granisetron and 250 mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2w g / mol), dissolve in 5 mL of dichloromethane, and mix the drug-containing PLGA with stirring The solution was added to 100 mL of the water phase containing 1% (w / v) stabilizer PVA to form an O / W emulsion, and the stirring was continued at room temperature for 6 h to volatilize the dichloromethane, wherein the stirring rate was 300-500 rpm. Stand still, and after the suspended matter sinks to the bottom, remove the supernatant, wash with water three times, collect microspheres by centrifugation, and freeze-dry.
Embodiment 2
[0038] Weigh 5 mg of granisetron and 250 mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2w g / mol), dissolve in 5 mL of dichloromethane, and mix the drug-containing PLGA with stirring The solution was added to 200 mL of water phase containing 1% (w / v) stabilizer PVA to form an O / W emulsion. Stirring was continued at room temperature for 6 h to volatilize dichloromethane, wherein the stirring rate was 300-500 rpm. Stand still, and after the suspended matter sinks to the bottom, remove the supernatant, wash with water three times, collect microspheres by centrifugation, and freeze-dry.
Embodiment 3
[0040] Weigh 5 mg of granisetron and 250 mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2w g / mol), dissolve in 5 mL of dichloromethane, and mix the drug-containing PLGA with stirring The solution was added to 50 mL of water phase containing 1% (w / v) stabilizer PVA to form an O / W emulsion. Stirring was continued at room temperature for 6 h to volatilize dichloromethane, wherein the stirring rate was 300-500 rpm. Stand still, and after the suspended matter sinks to the bottom, remove the supernatant, wash with water three times, collect microspheres by centrifugation, and freeze-dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com