Patents
Literature
135 results about "Skin patch" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug delivery method
Analyte measurement
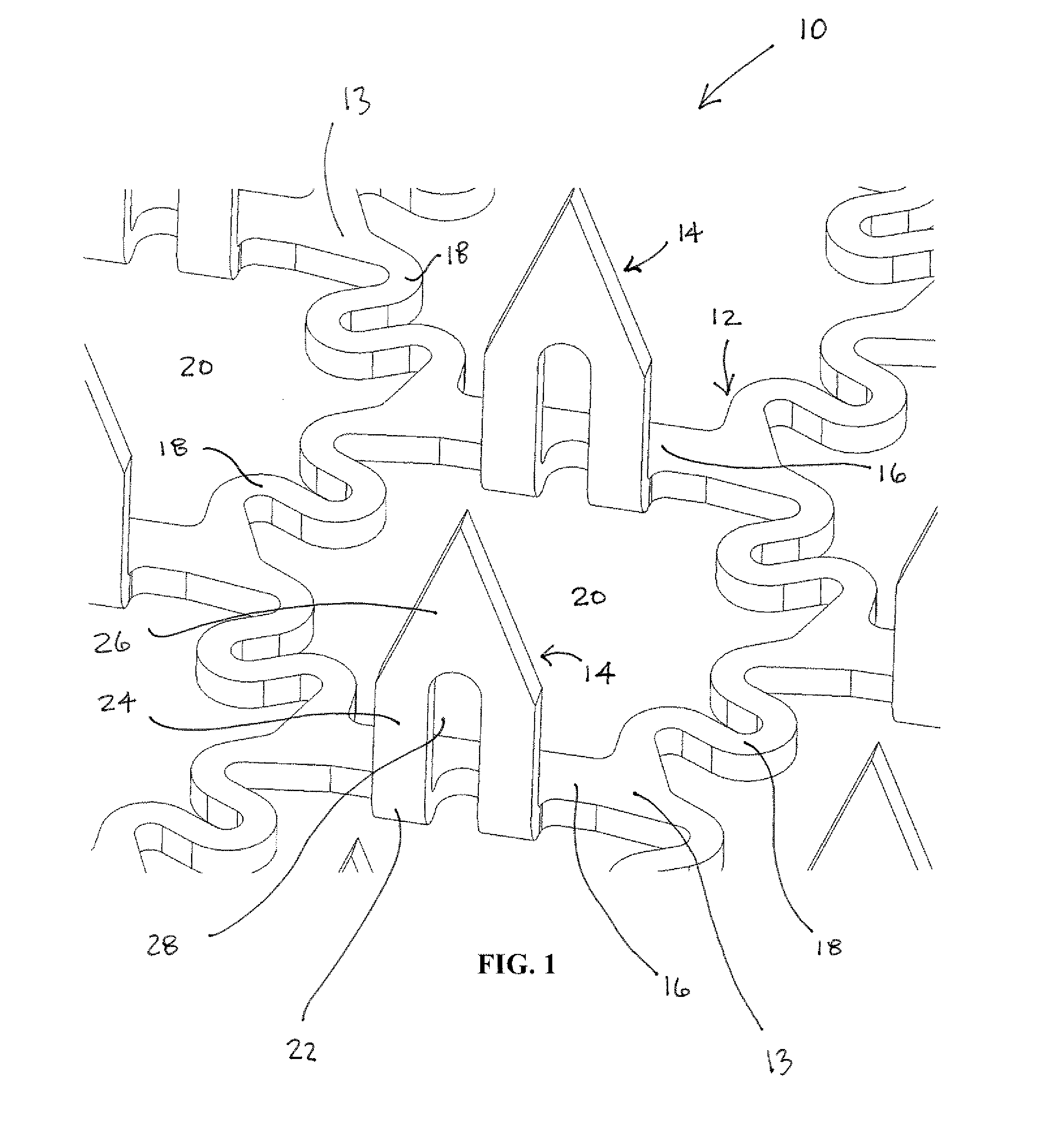
InactiveUS20040096959A1Low viscosityMore suitedBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical detectorAnalyte
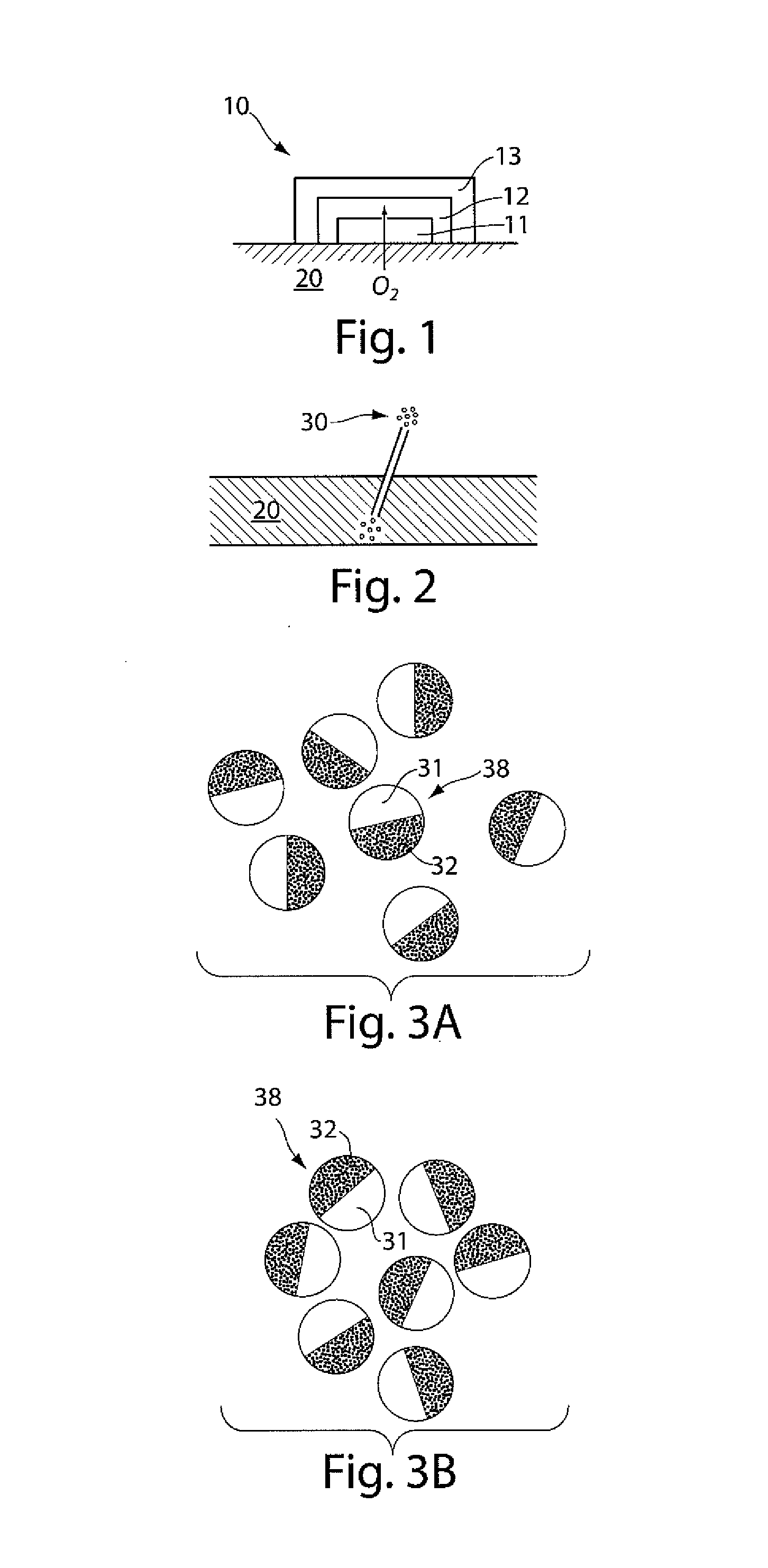
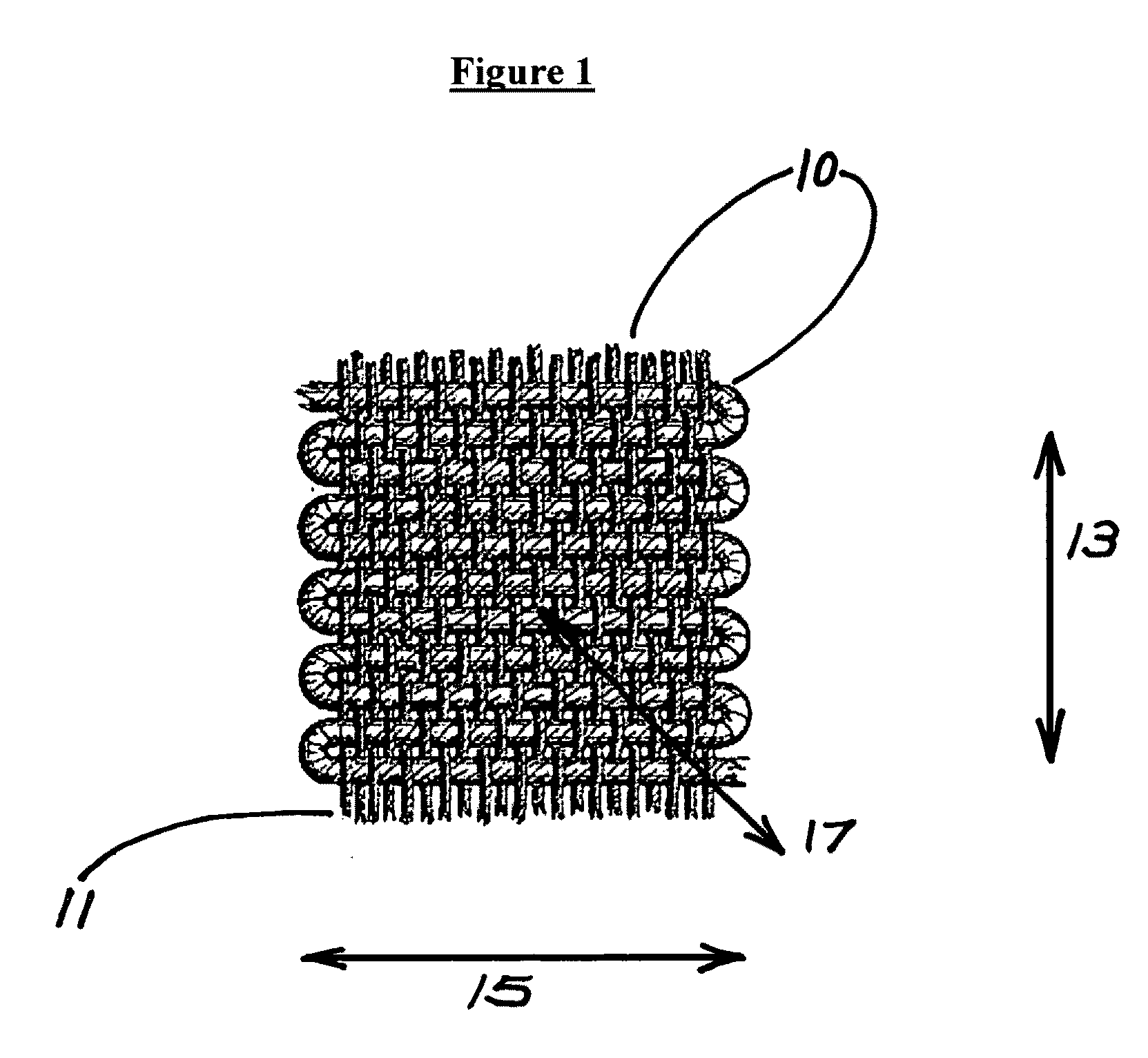
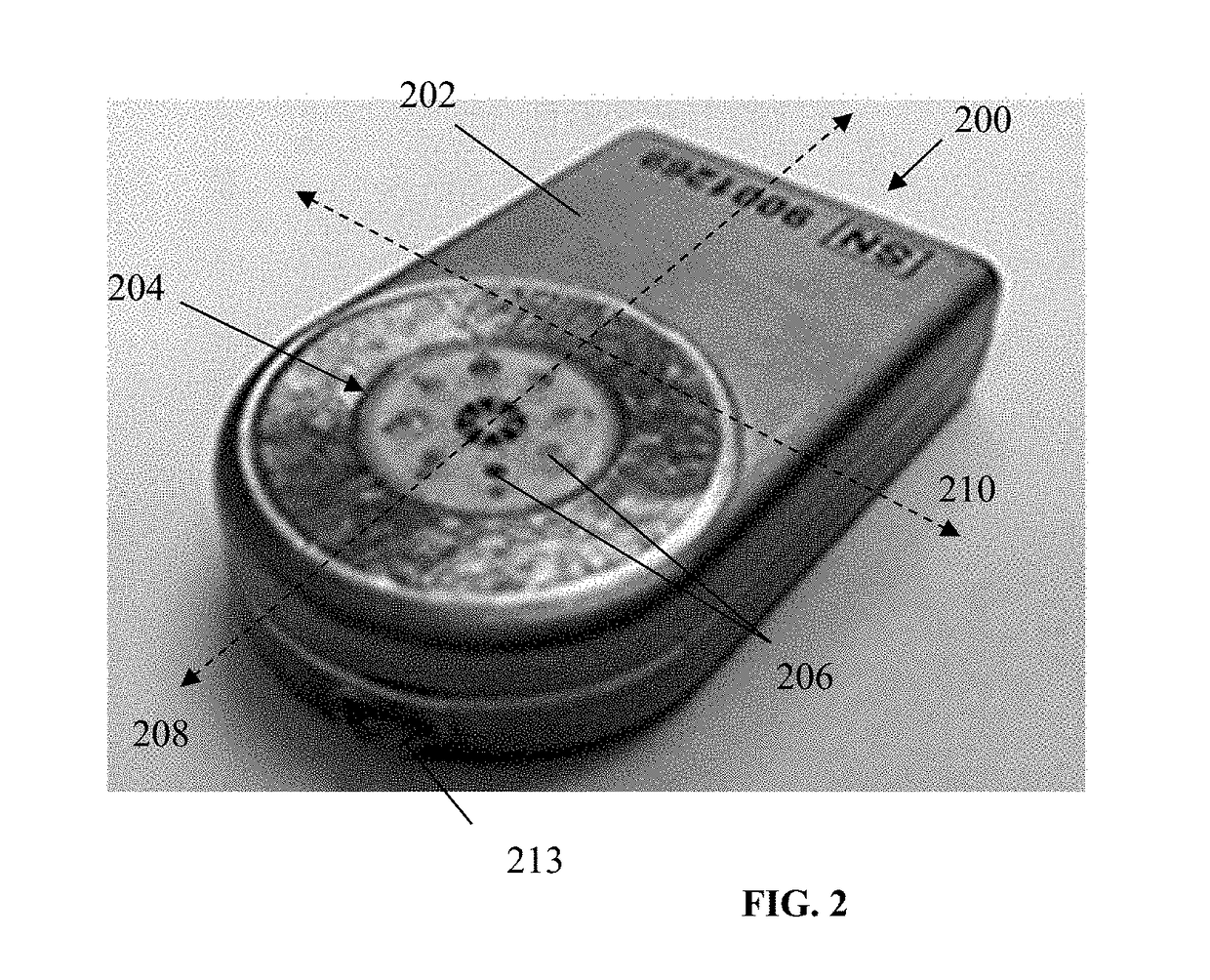
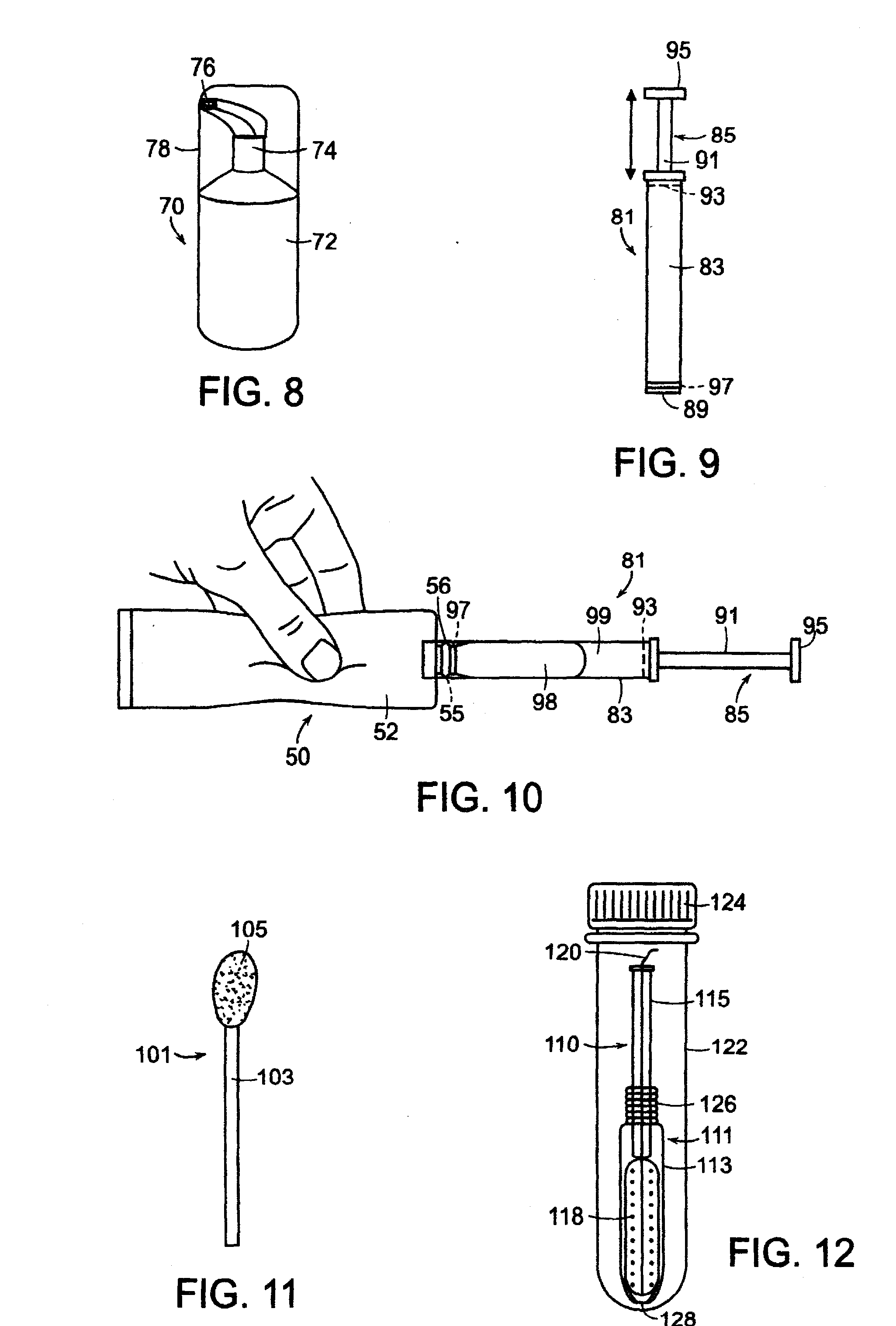
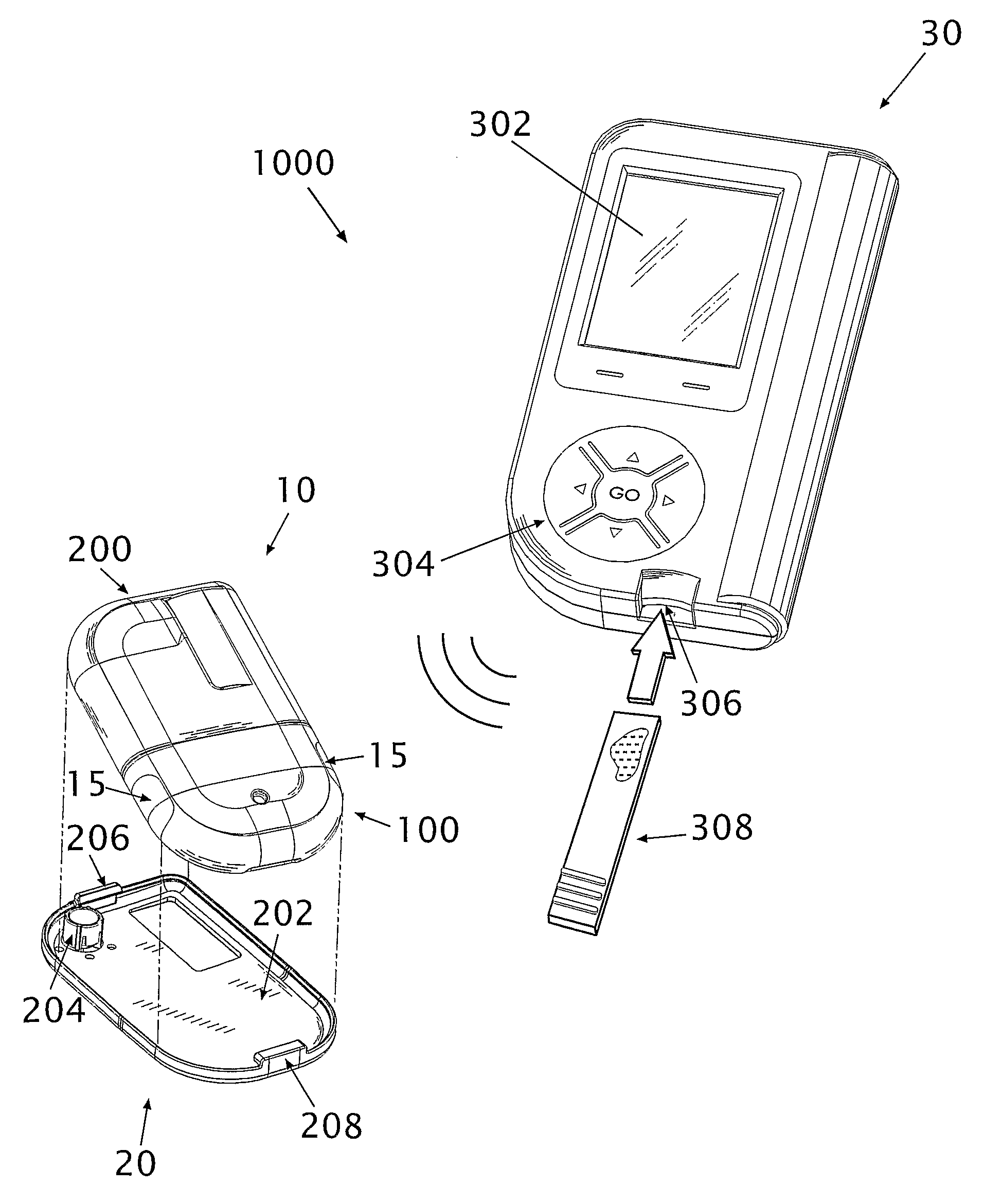
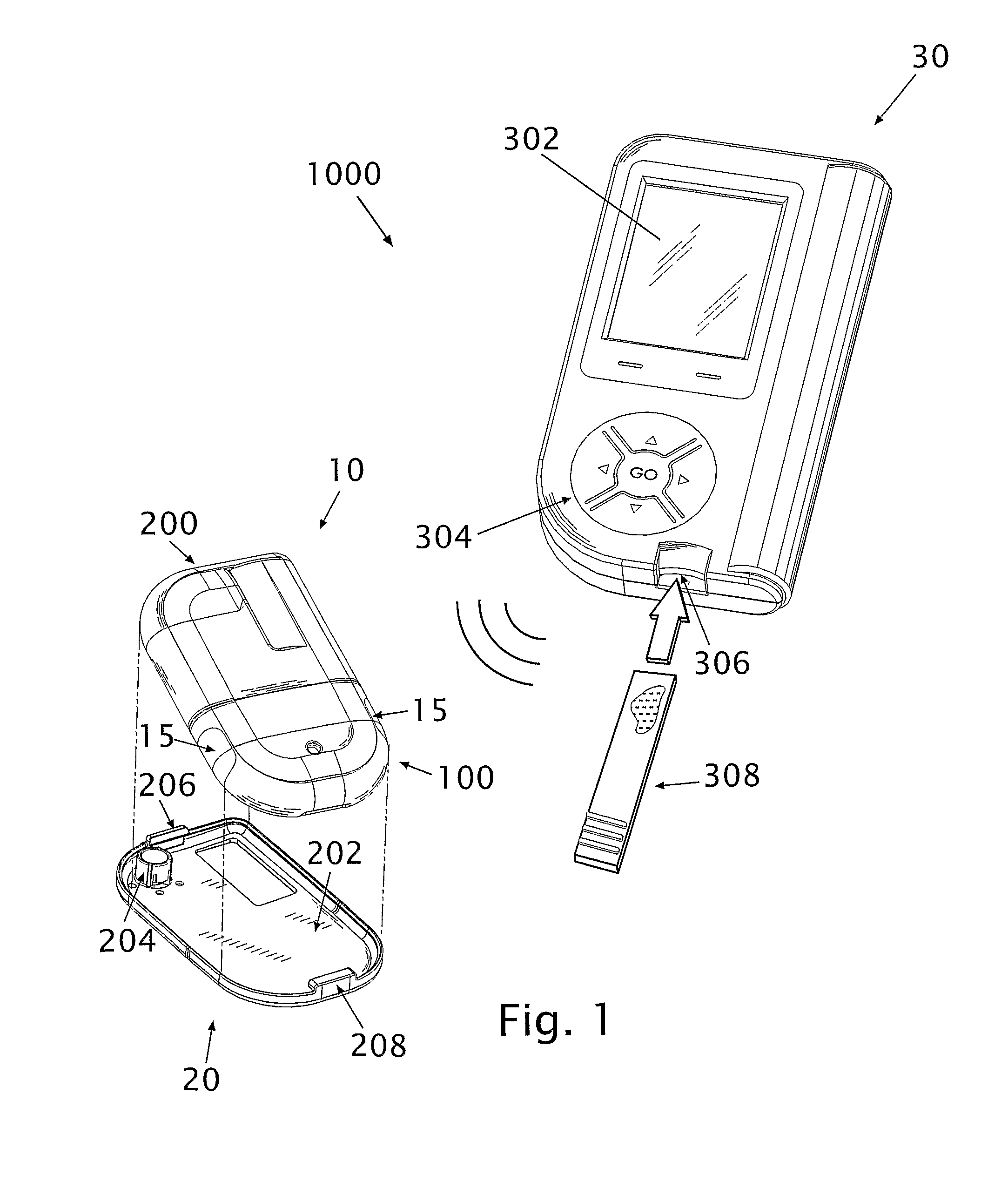
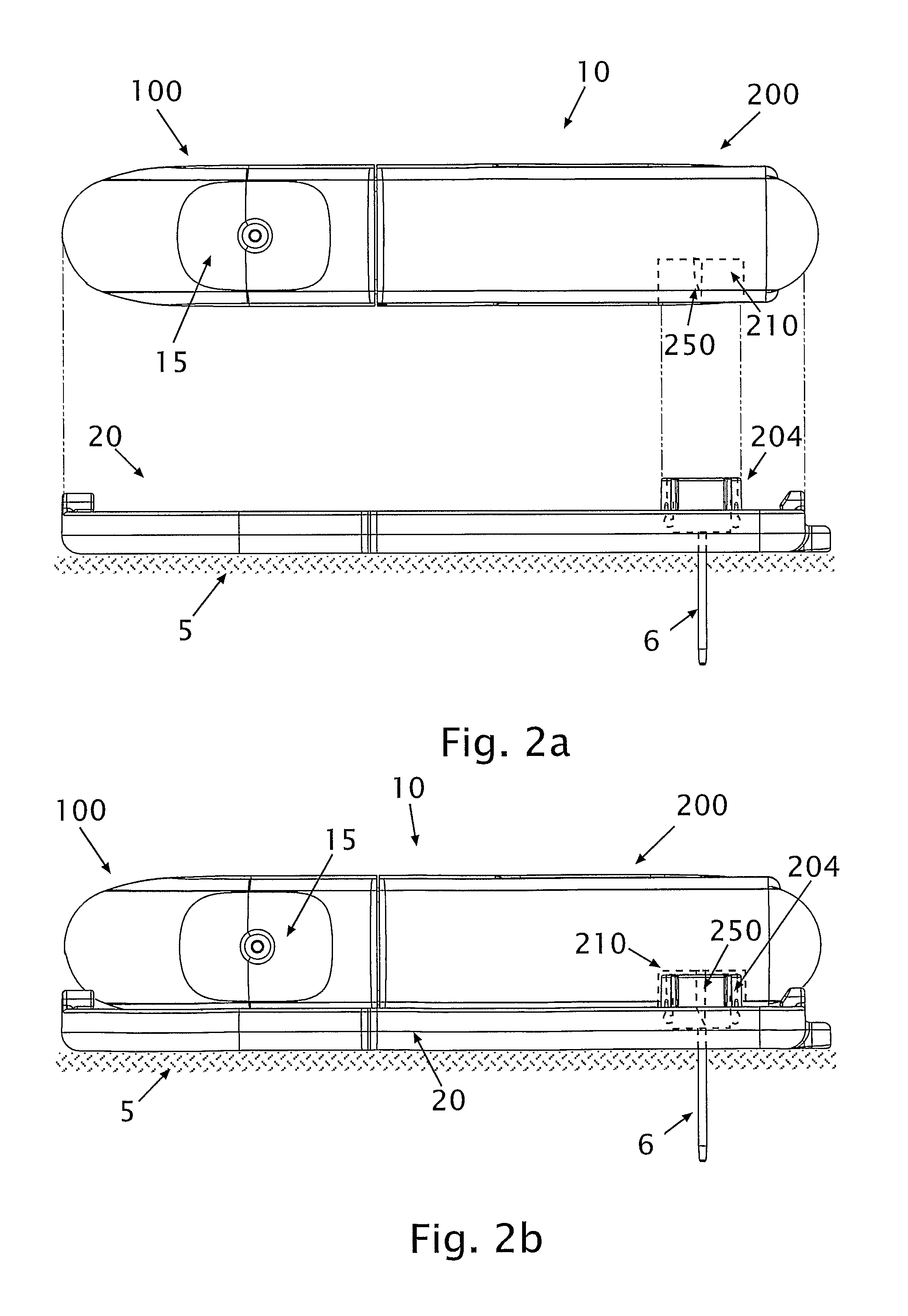
A glucose sensor in the form of a skin patch 2 has a microneedle 4 which painlessly penetrates the skin to draw out interstitial fluid. The interstitial fluid passes to a common entrance port 7. A series of microchannels 8 is provided on the skin patch. The fluid drawn onto the patch is selectively switched between a number of microchannels 8 by means of electro-osmotic pumps 10 and hydrophobic gates 12. Each microchannel 8 has an electrochemical detector 11 for sensing gluocse concentration. Also disclosed is a monlithic device with an integrated lance 83.
Owner:LIFESCAN IP HLDG LLC +1
Devices, methods, and kits for non-invasive glucose measurement
InactiveUS20060004271A1Minimize impactMicrobiological testing/measurementSurgeryMeasurement deviceDisplay device
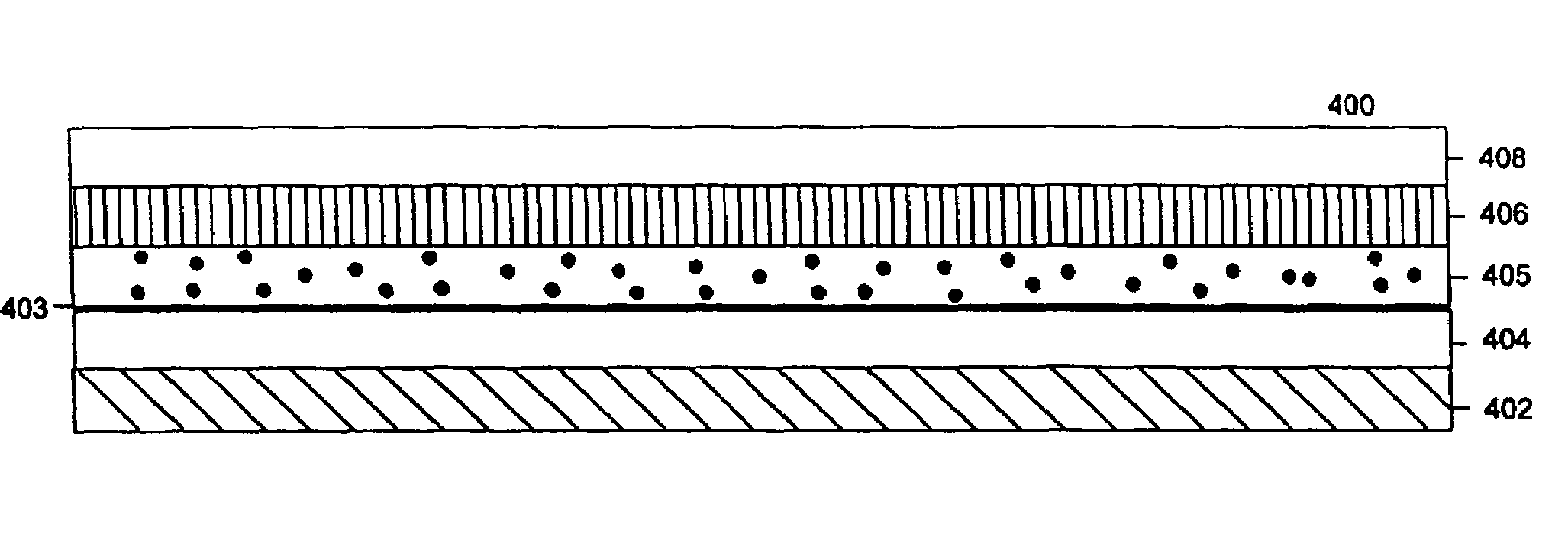
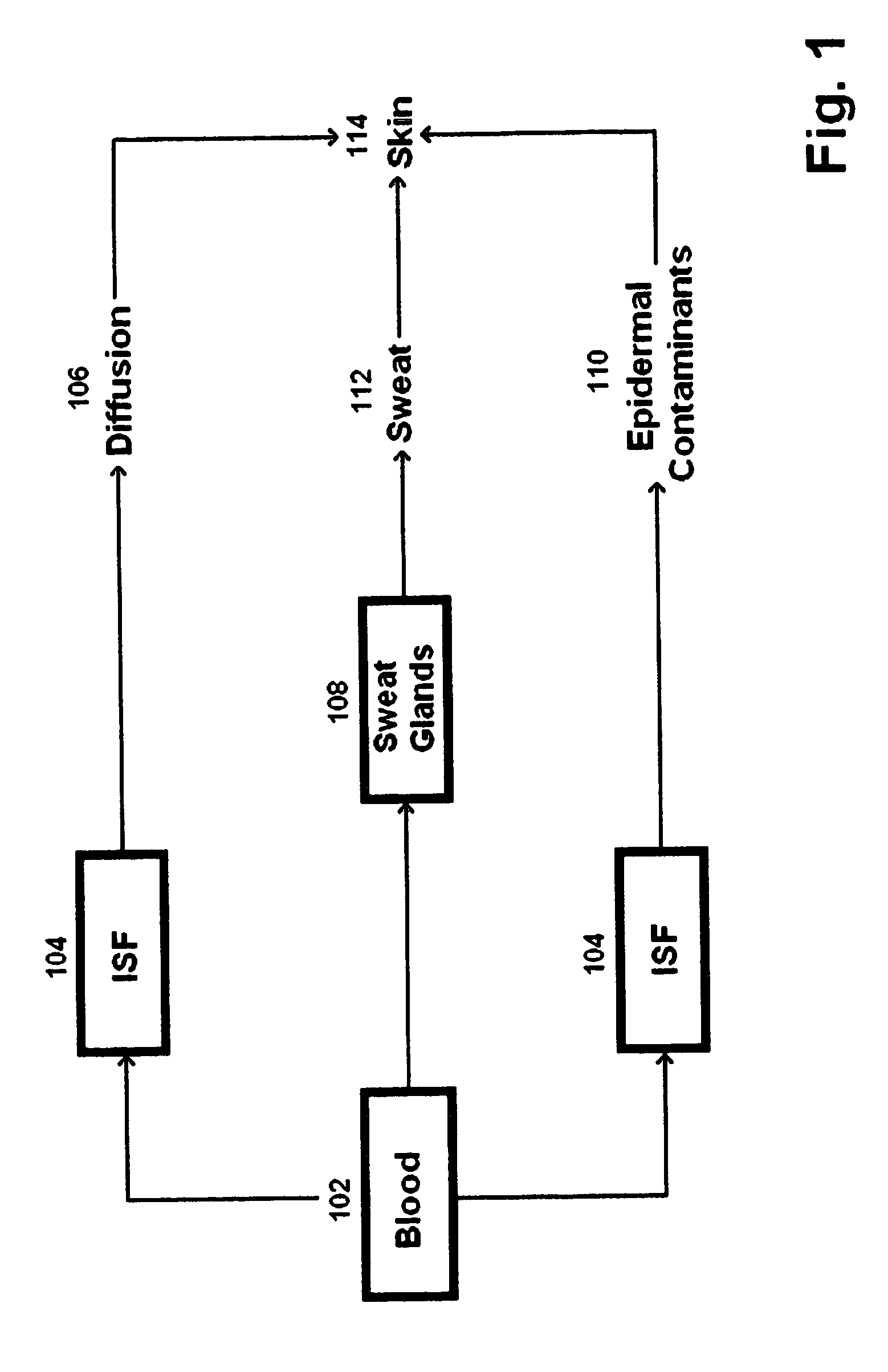
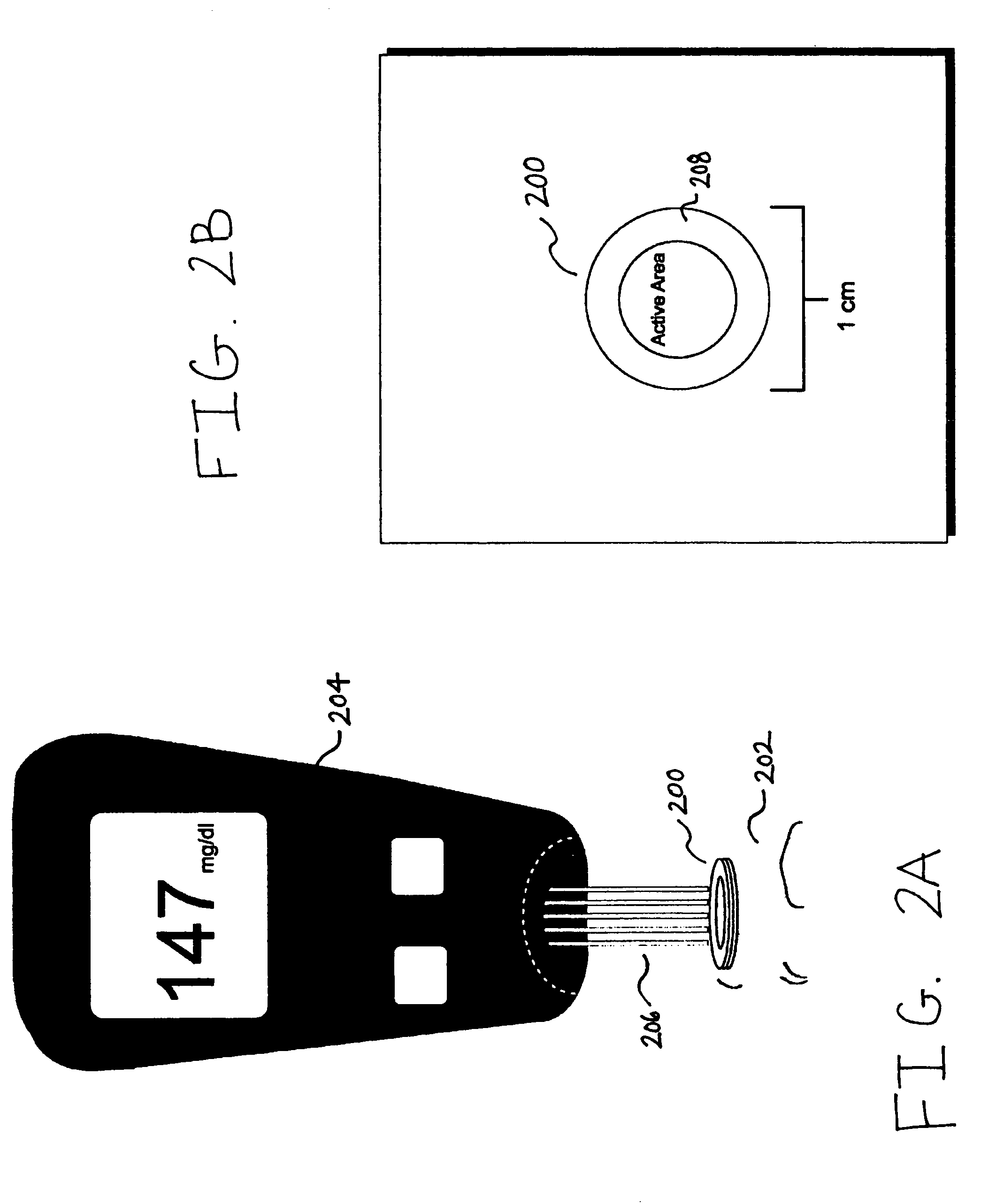
Described are devices, methods, and kits for non-invasively measuring glucose. In general, the devices comprise skin patches for placement on a skin surface and measurement devices for measuring glucose collected in the patches. The patches may include an adhesive material, a collection layer, an interface layer, and a sweat-permeable membrane. The sweat-permeable membrane is configured to act as a barrier to epidermal contaminants and glucose brought to the skin surface via diffusion. In this way, non-correlatable skin surface glucose will not be measured. The patches may further include components to induce a local sweat response. The measurement device typically includes a display, a processor, and a measurement mechanism. The methods typically include the steps of wiping the skin surface with a wipe containing at least one solvent for removing glucose, placing a patch on a skin surface, and measuring glucose collected in the patch. Kits comprising the patch and measurement device are also described.
Owner:VIVOMEDICAL INC
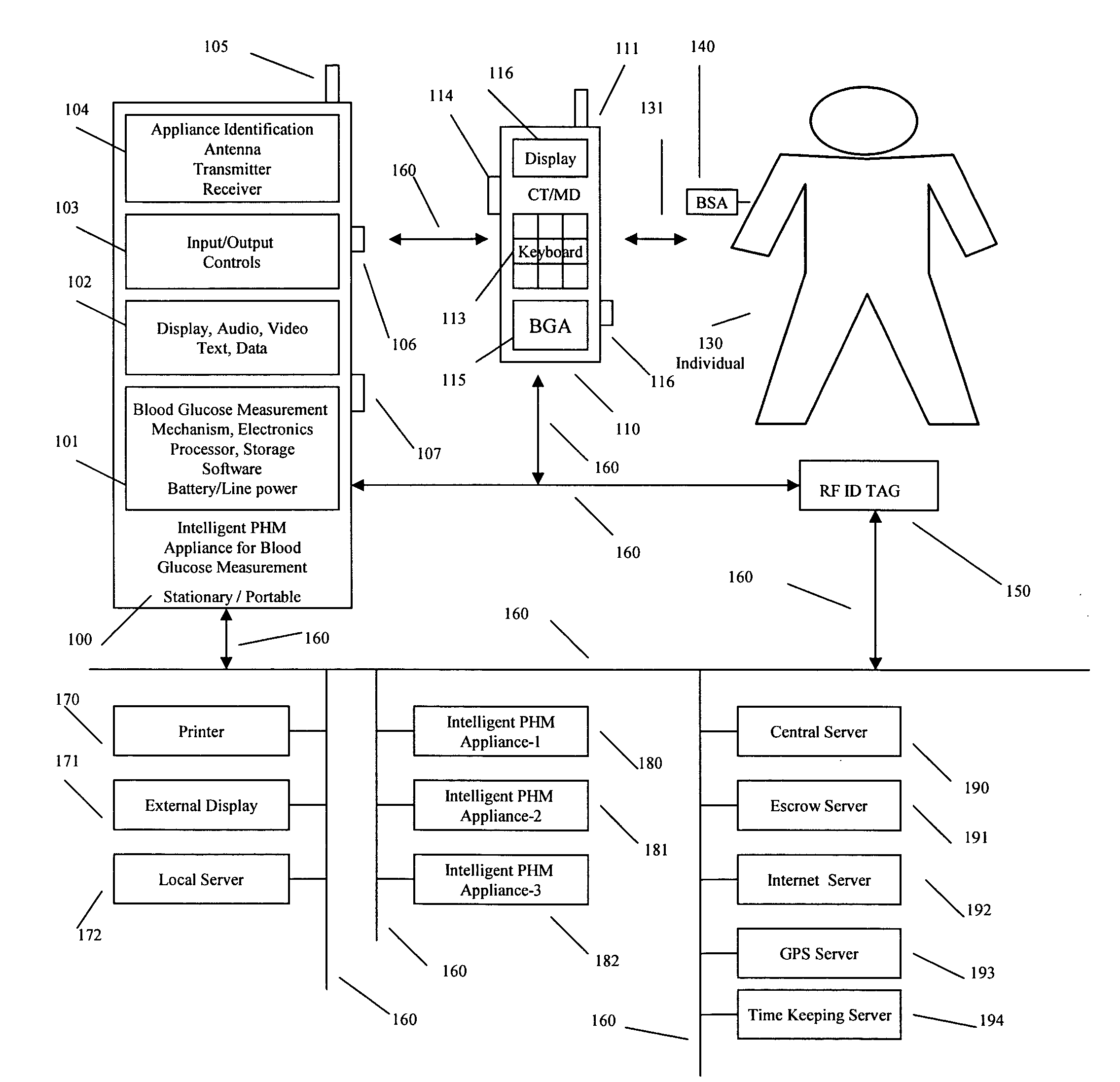
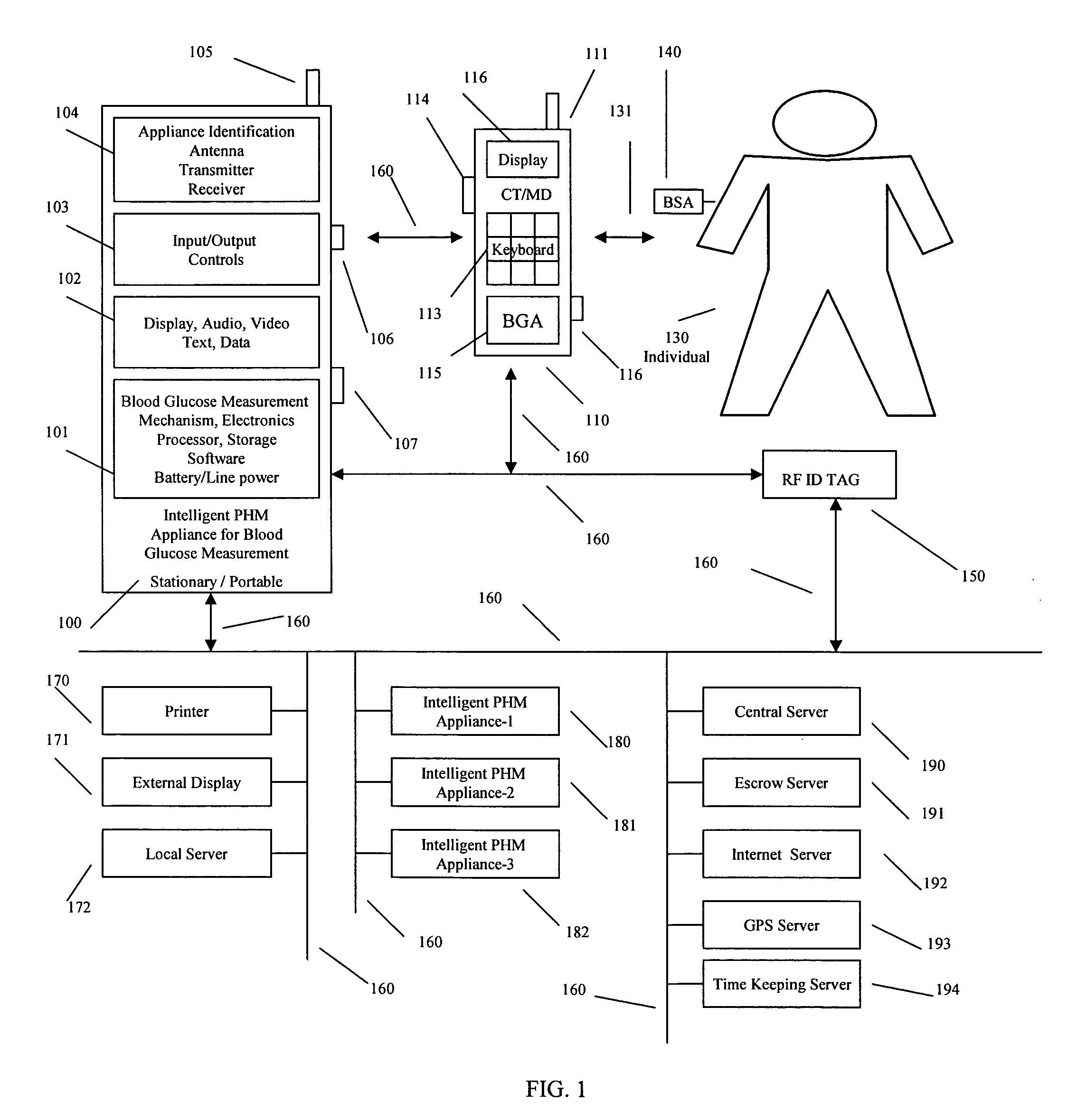
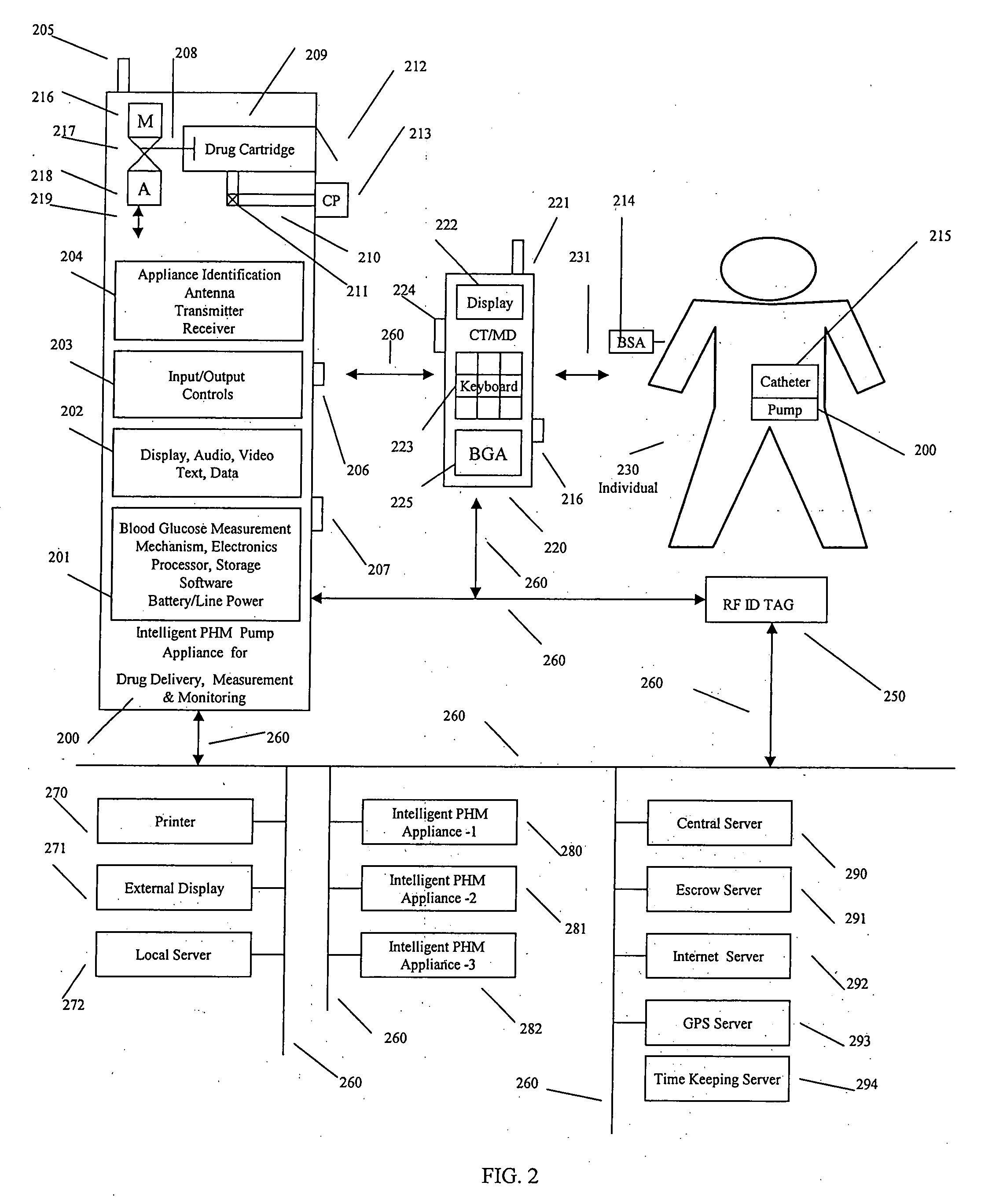
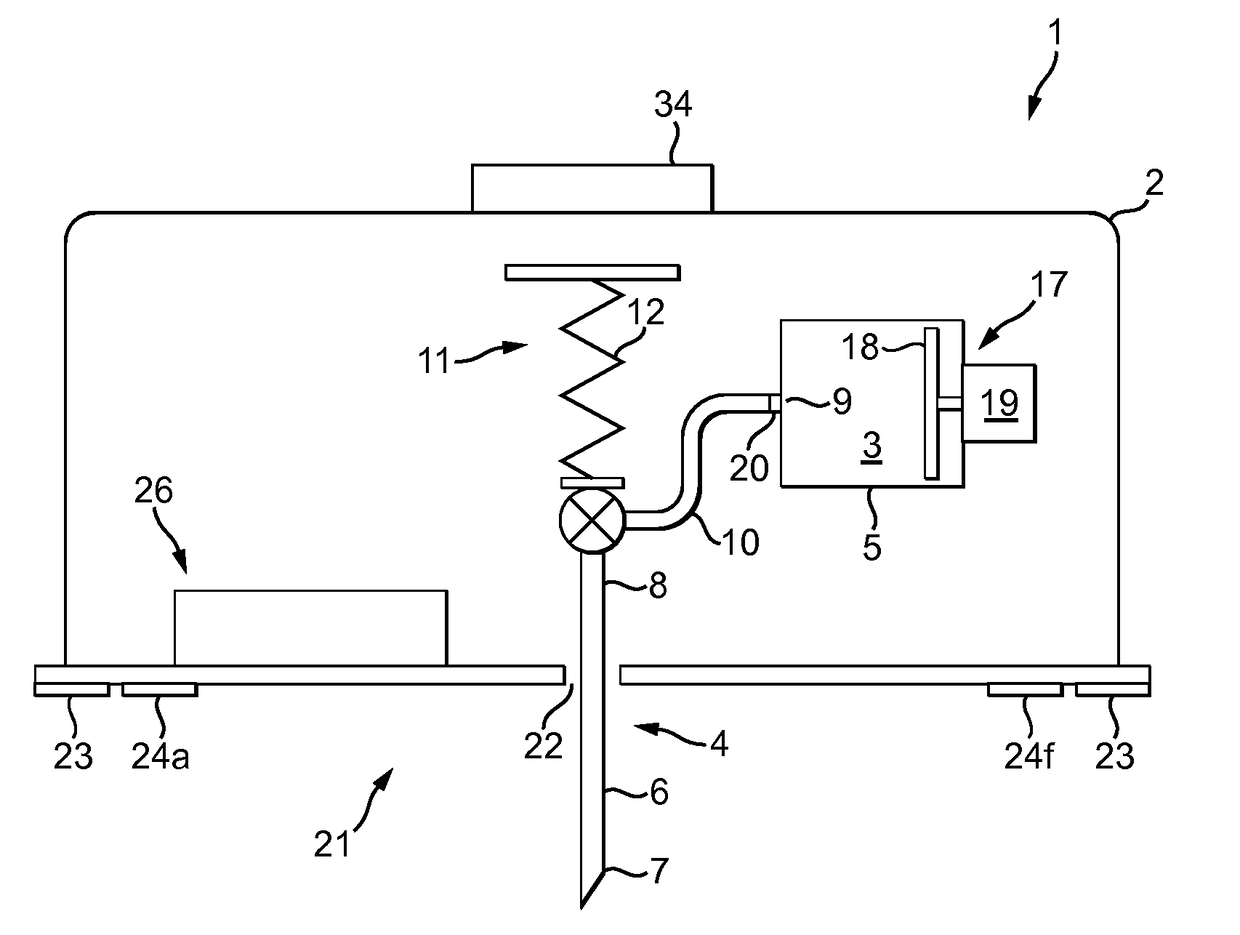
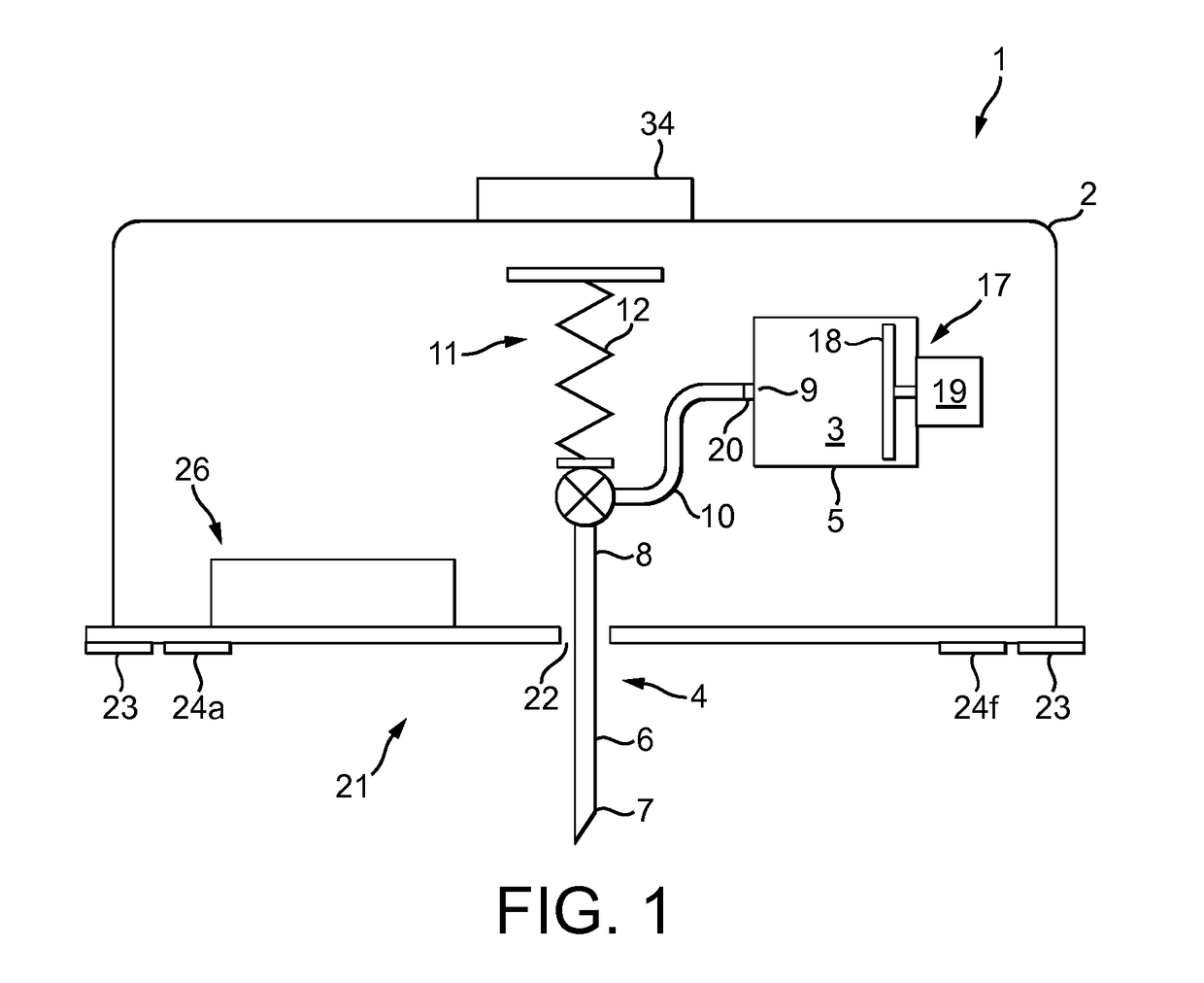
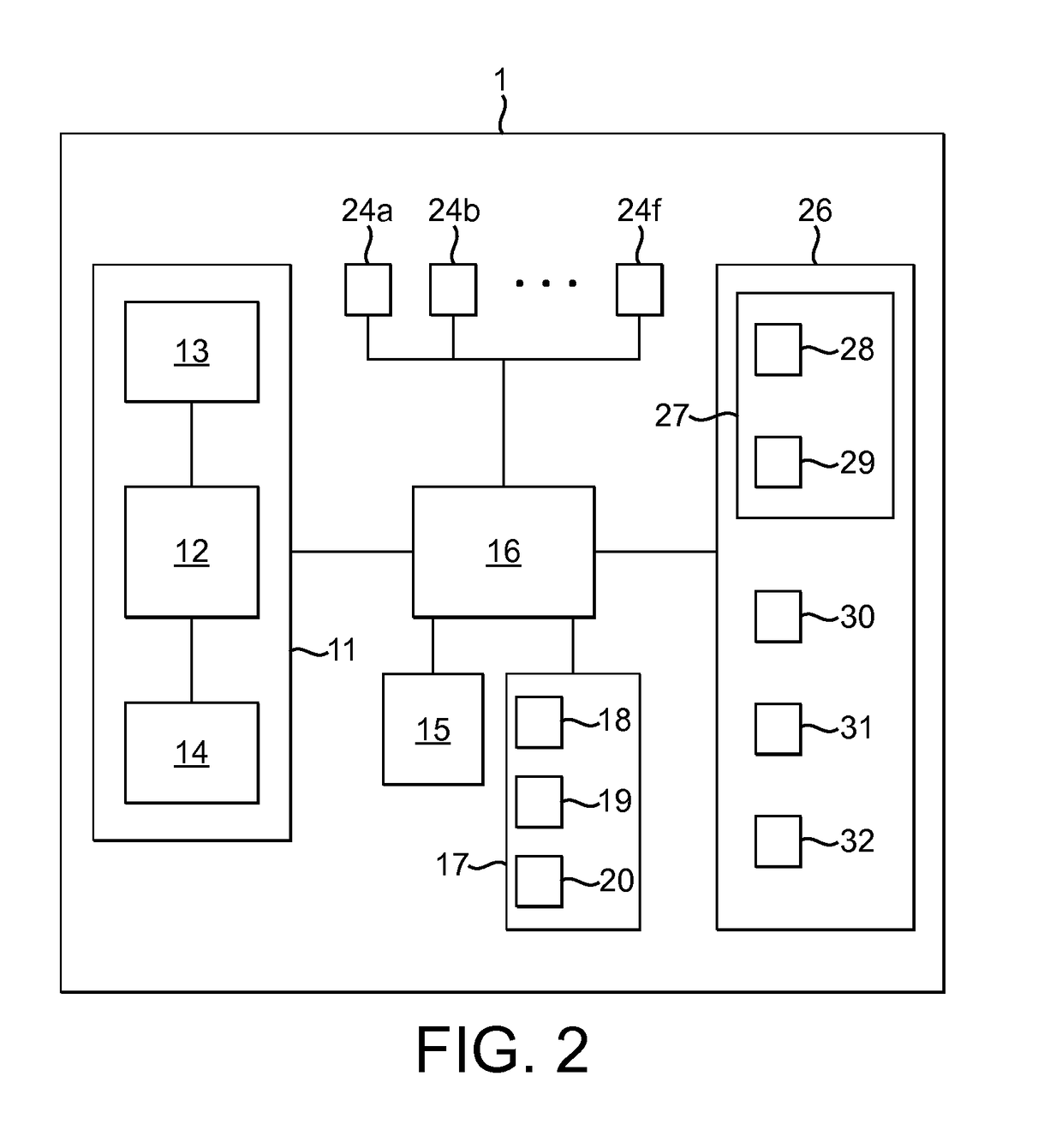
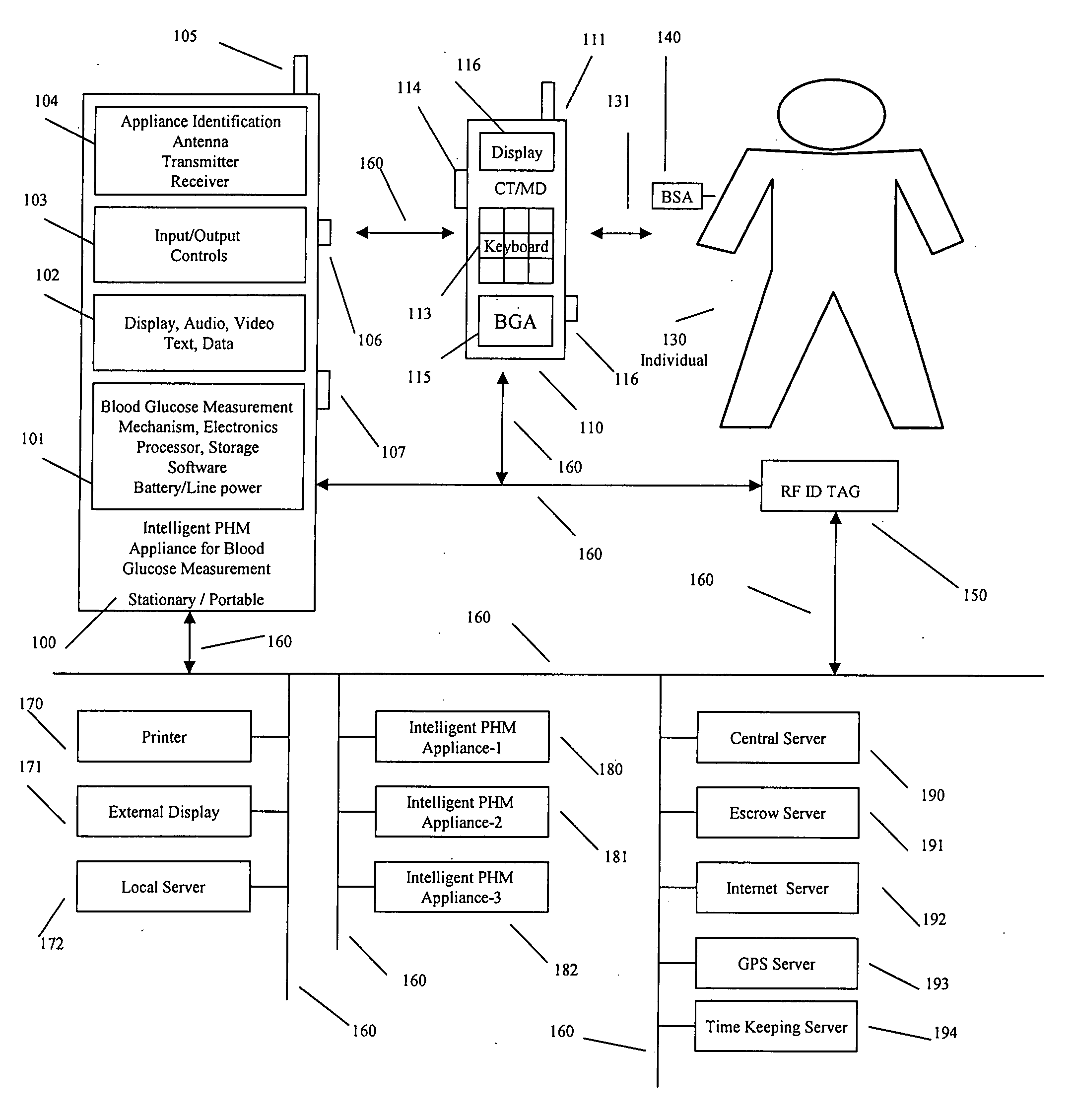
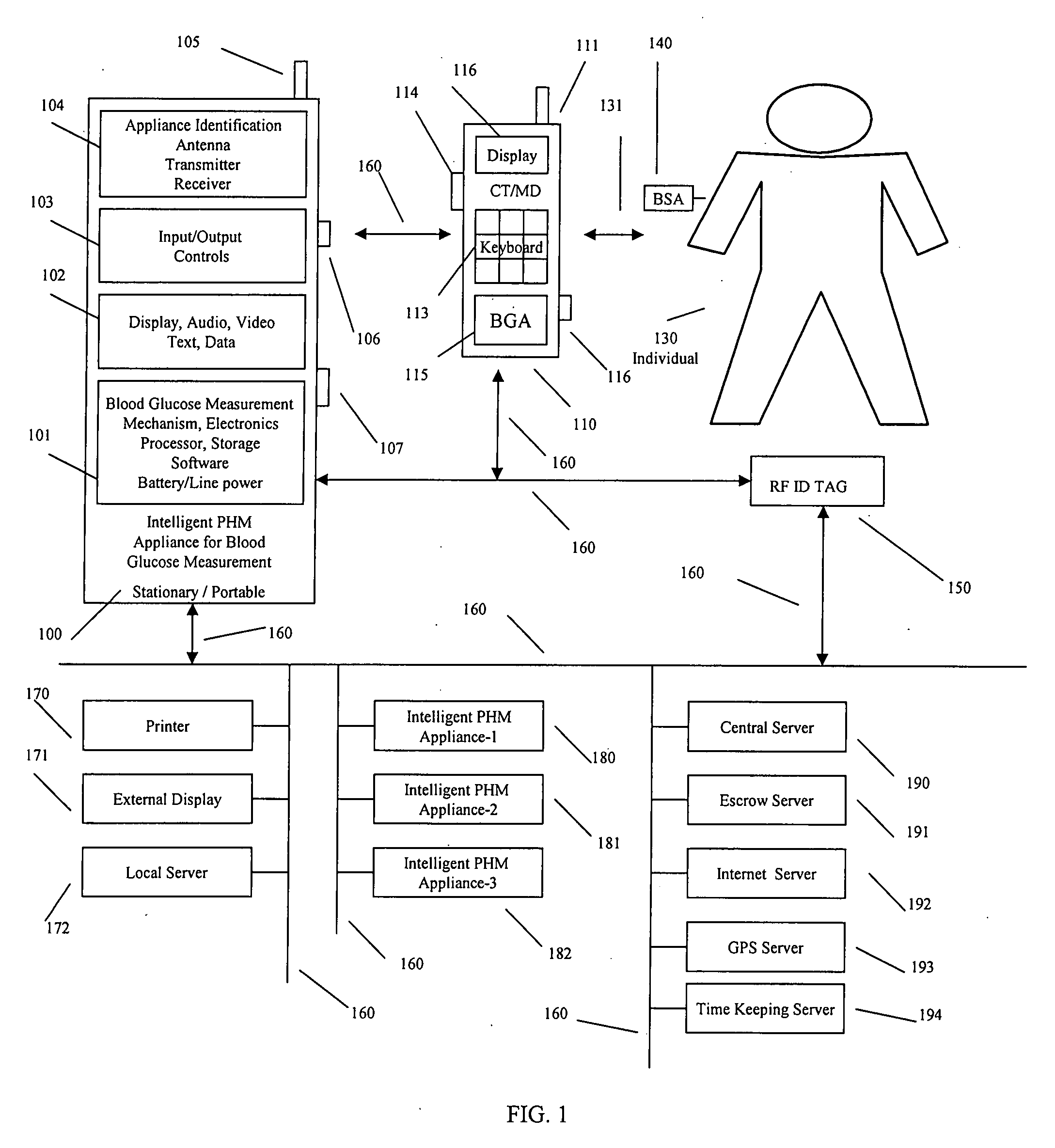
Intelligent personal health management appliances for the measurement and monitoring of health factors and controlled delivery of drugs
InactiveUS20080139907A1Precise positioningPointing accuratelyPhysical therapies and activitiesElectrotherapyDiseaseDiabetes mellitus
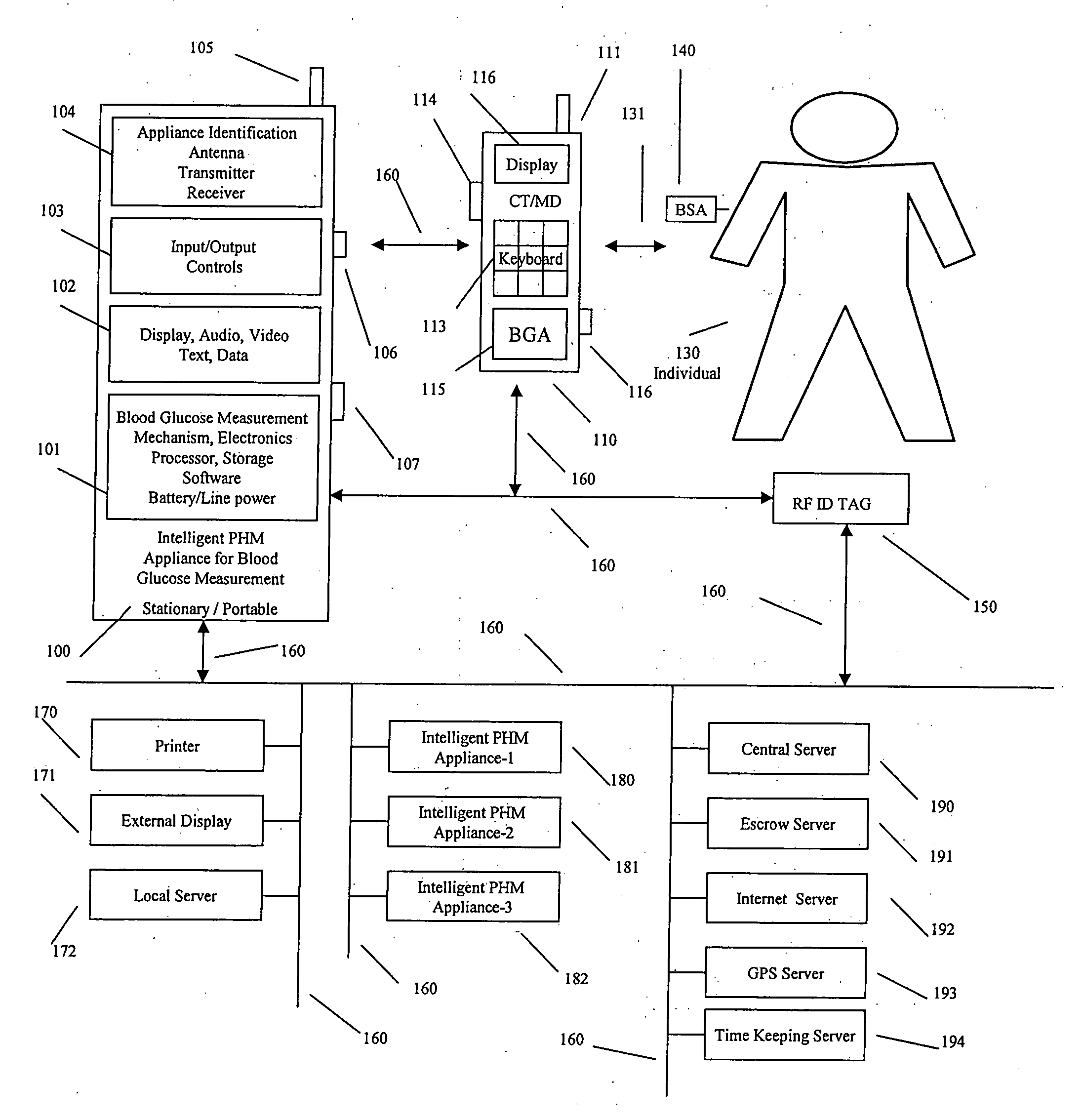
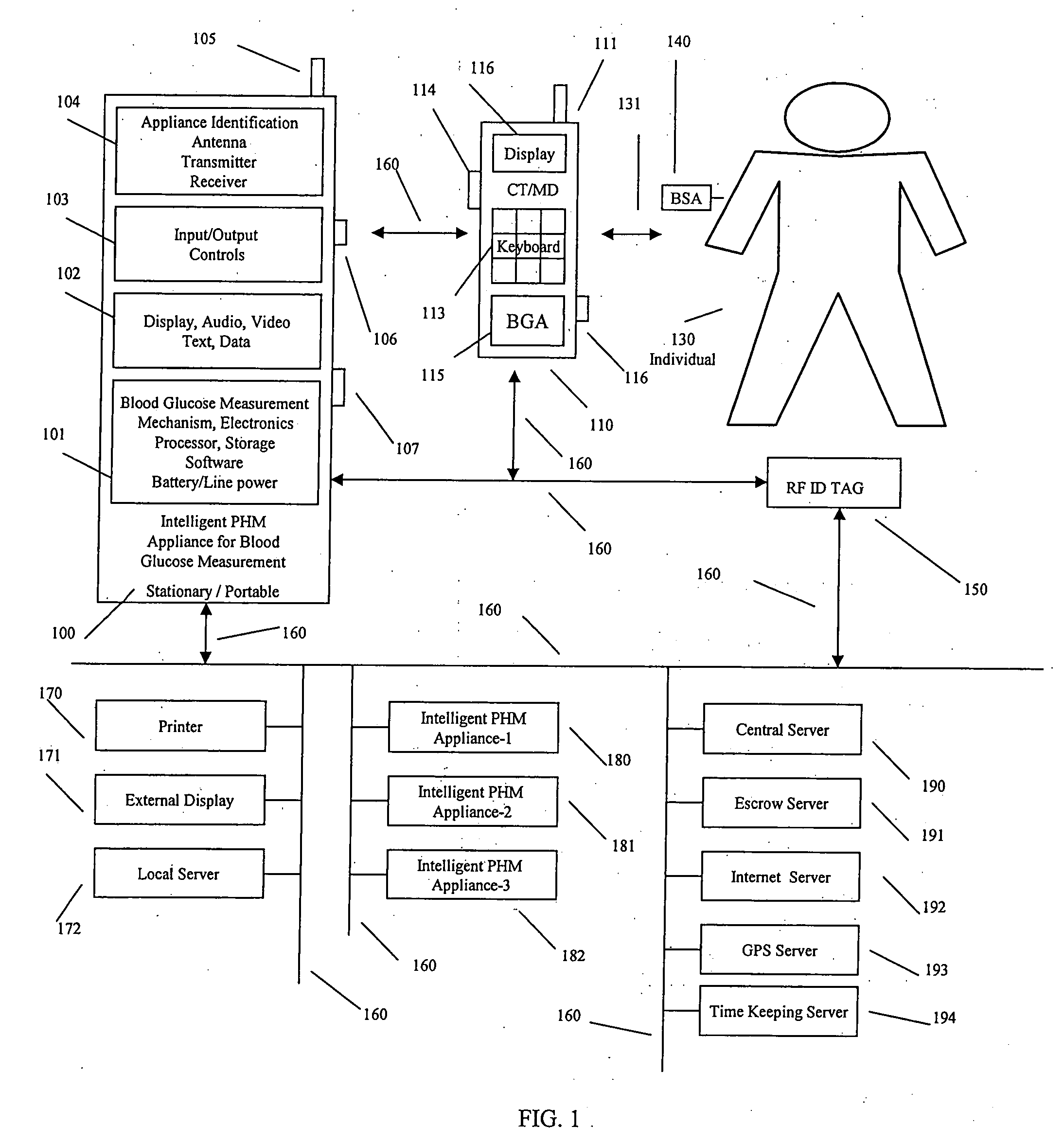
A system, method and apparatus for real time measurement and monitoring of various personal health parameters including controlled delivery of drugs / medications by intelligent pump appliances, intelligent inhalation appliances and intelligent skin patch appliances used in a standalone manner or in a wired or wireless networked configuration, in conjunction with various peripheral devices, other intelligent appliances, servers, RF ID Tags and stationary / mobile devices. The intelligent appliances relate to the measurement, monitoring and delivery of insulin / other drugs for the treatment of diabetes and other diseases. The method also additionally includes the application of intelligent appliances for pain management including visualization of organs and body locations exhibiting pain.
Owner:IP HLDG LLC
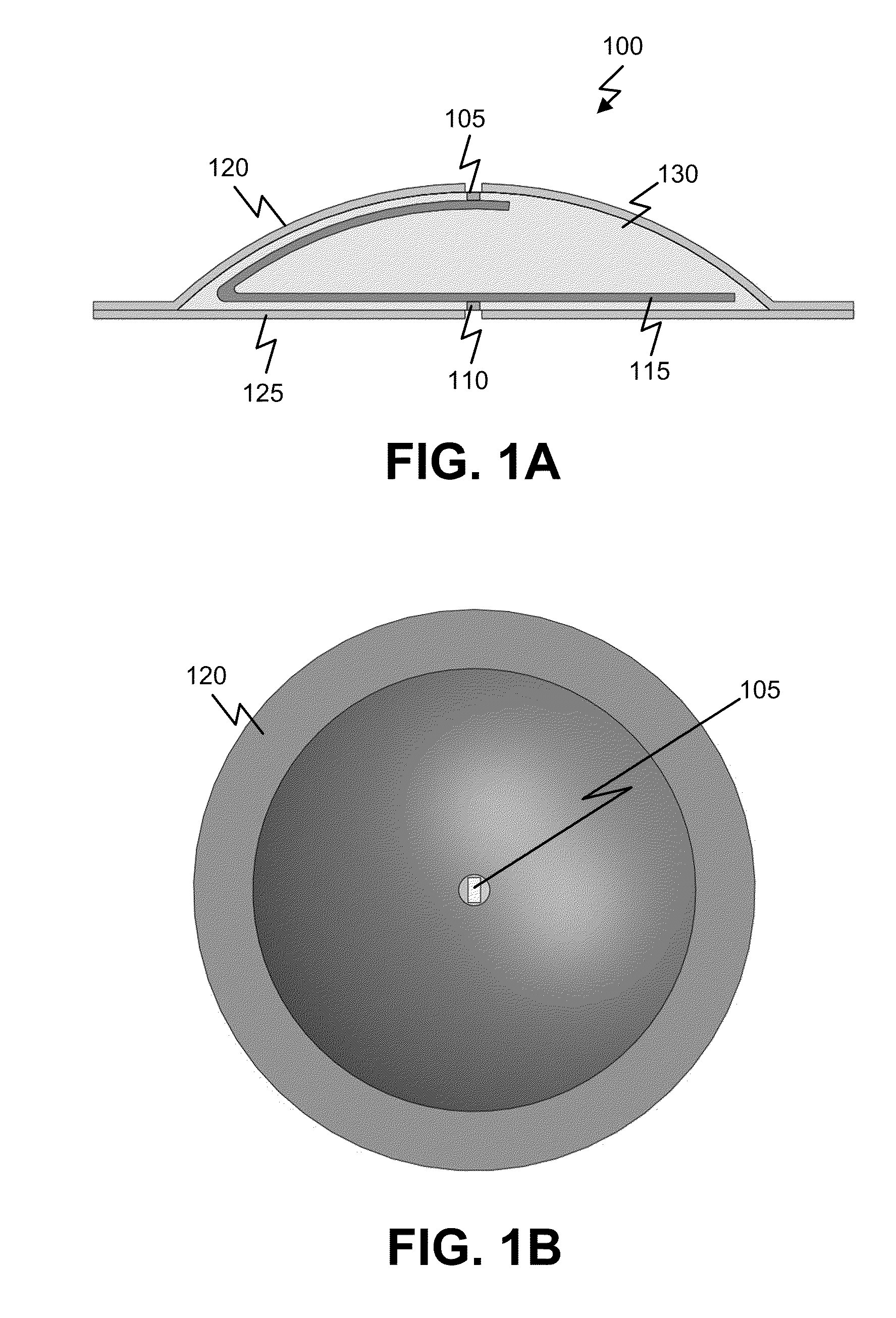
Tissue Conforming Microneedle Array and Patch For Transdermal Drug Delivery or Biological Fluid Collection
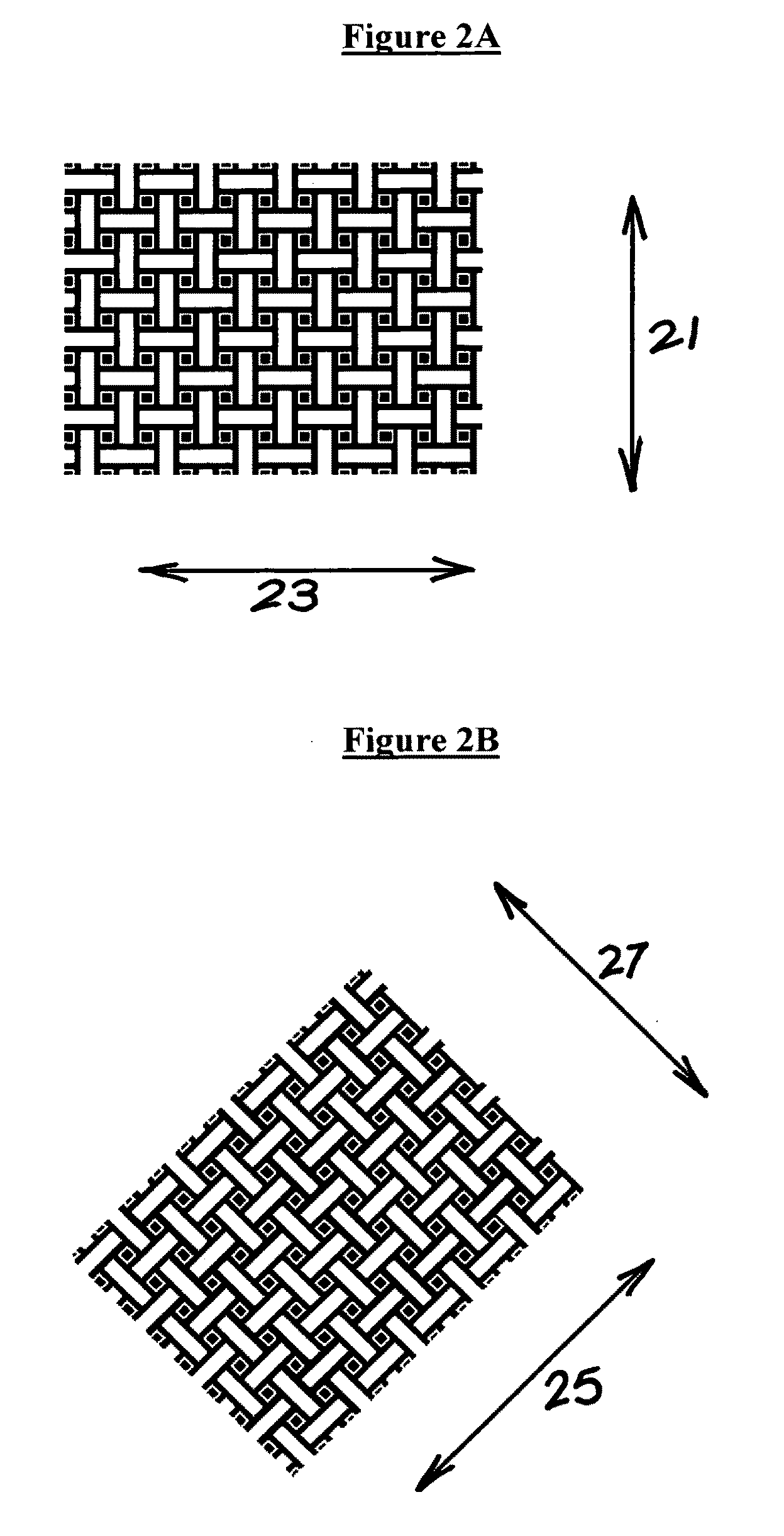
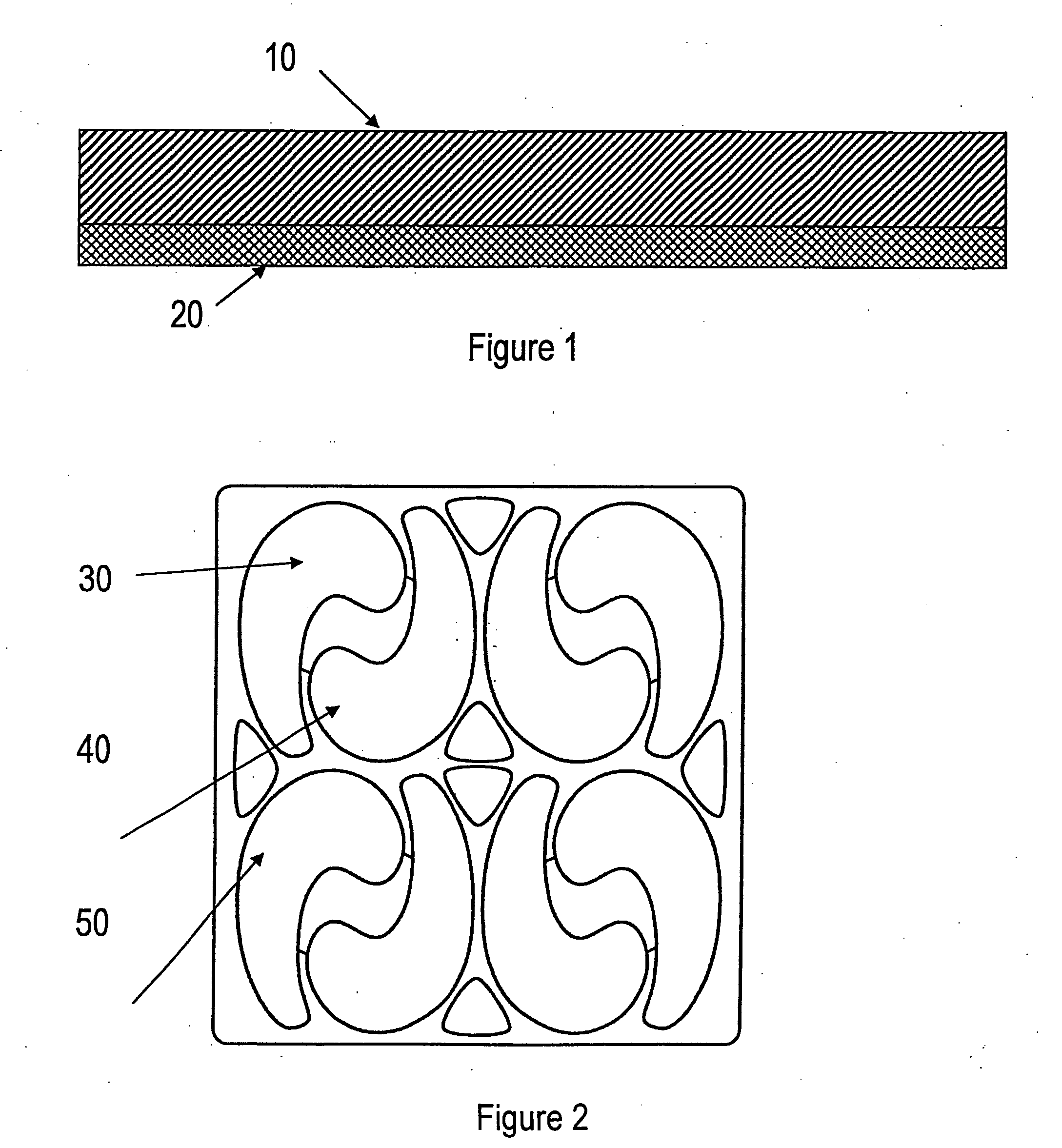
Microneedle arrays are provided for use on a contoured or flexible tissue surface. In one embodiment, the microneedle array includes a plurality of microneedles, each having a base portion, a tip end portion distal to the base portion, and body portion therebetween; and a flexible substrate which comprises a plurality of apertures, each of which are defined by (i) a plurality of substrate elements which are integral with the base portions of the microneedles, and (ii) at least one spring element connecting at least two of the substrate elements. The spring element may include a curved element, such as a C-shaped, U-shaped, or S-shaped element. Apertures may be defined, for example, by two substrate elements, which connected to three or four spring elements. A skin patch is provided for therapeutic or diagnostic applications, which includes the microneedle array and an adhesive material.
Owner:YUZHAKOV VADIM V
Compositions and methods
Non-aerosol spray-on skin patch compositions as described comprising at least one substantially water insoluble film forming agent, at least one film plasticizer agent, at least one water soluble compound, and at least one organic solvent, the composition forming a flexible, porous and physiologically compatible skin patch when sprayed on to skin and allowed to dry. Also described are methods of improving wound healing by administering a physiologically active ingredient to a patient in need of such treatment.
Owner:KO THOMAS SAI YING
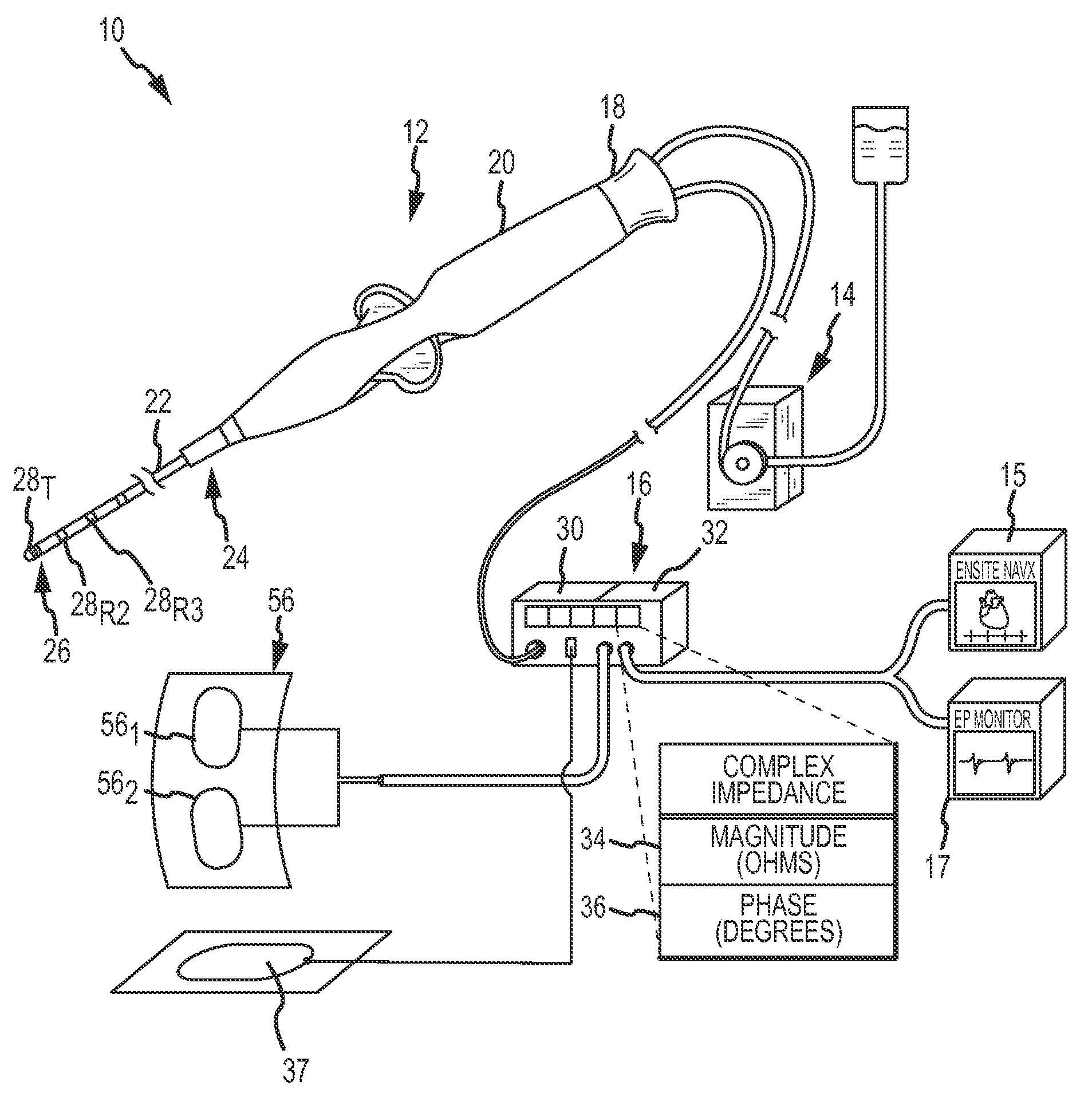
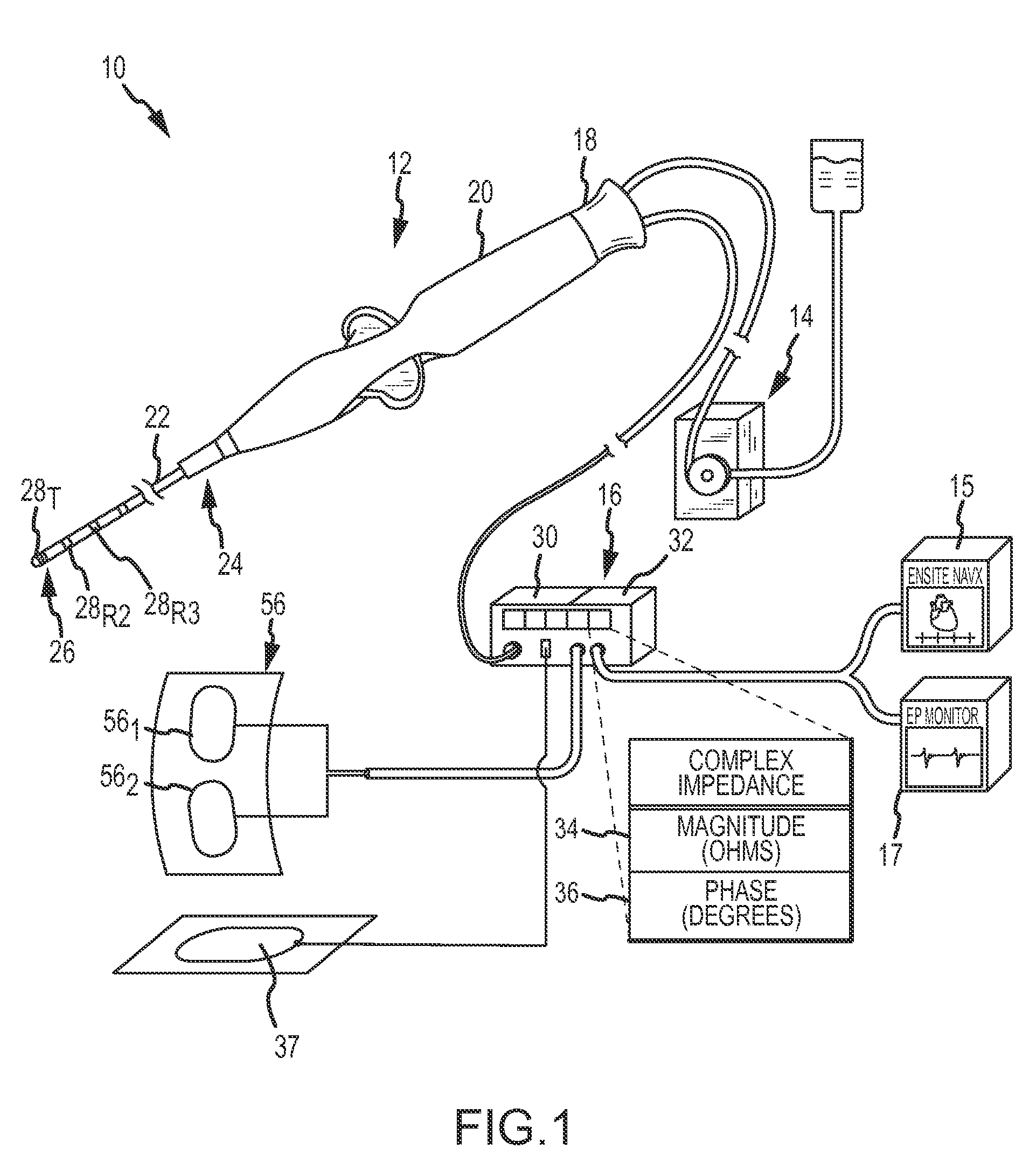
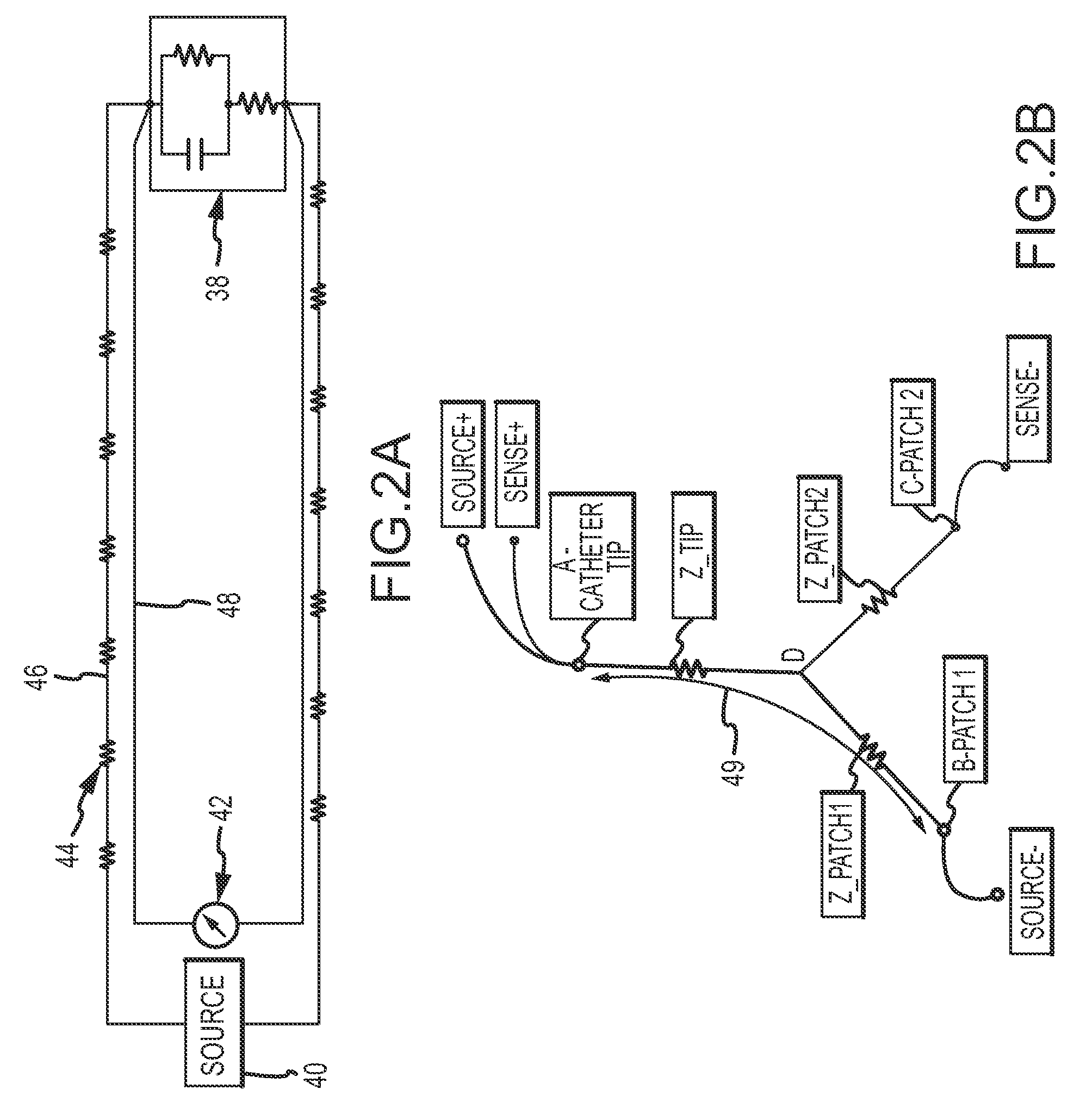
System and method for measurement of an impedance using a catheter such as an ablation catheter
ActiveUS20090171345A1Suitable for useMaximize predetermined distanceSurgical instruments for heatingRf ablationPatch electrode
A catheter and patch electrode system is provided for use with an apparatus, such as an ablation generator, having a 4-wire interface for improved impedance measurement. The 4-wire interface includes a pair of source connectors across which an excitation signal is produced and a pair of sense connector wires across which the impedance is measured. The RF ablation generator may also produce an ablation signal across a source wire and an indifferent return patch electrode. The system further includes a cable that connects the generator to a catheter. The catheter includes a shaft having a proximal end and a distal end, with an ablation tip electrode disposed at the distal end. A source lead is electrically coupled to the tip electrode and extends through the shaft to the proximal end where it is terminated. An optional sense lead is also electrically coupled to the tip electrode and extends through the shaft to the proximal end. The system further includes a source return (e.g., skin patch) and a sense return (e.g., skin patch), either or none of which may be combined with the indifferent return, and if used may be placed on opposite sides of the patient for improved performance. The impedance sensor circuit produces an excitation signal across the source connectors, which is then carried to the catheter by the cable, then to the tip electrode, travels through the complex load (tissue volume), and returns to the generator via a patch electrode. The impedance is measured by observing the voltage drop across the sense connectors caused by the excitation signal.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
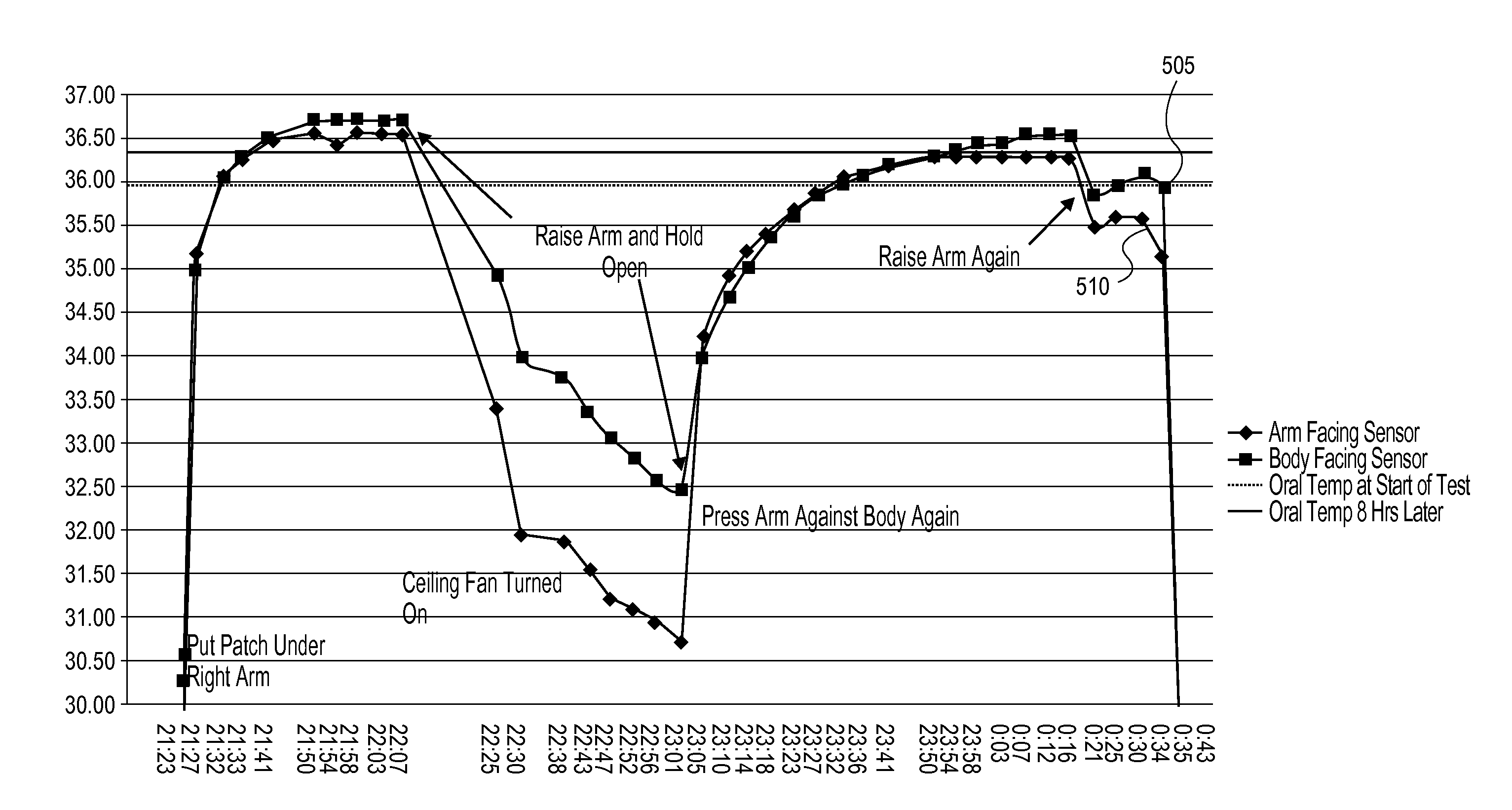
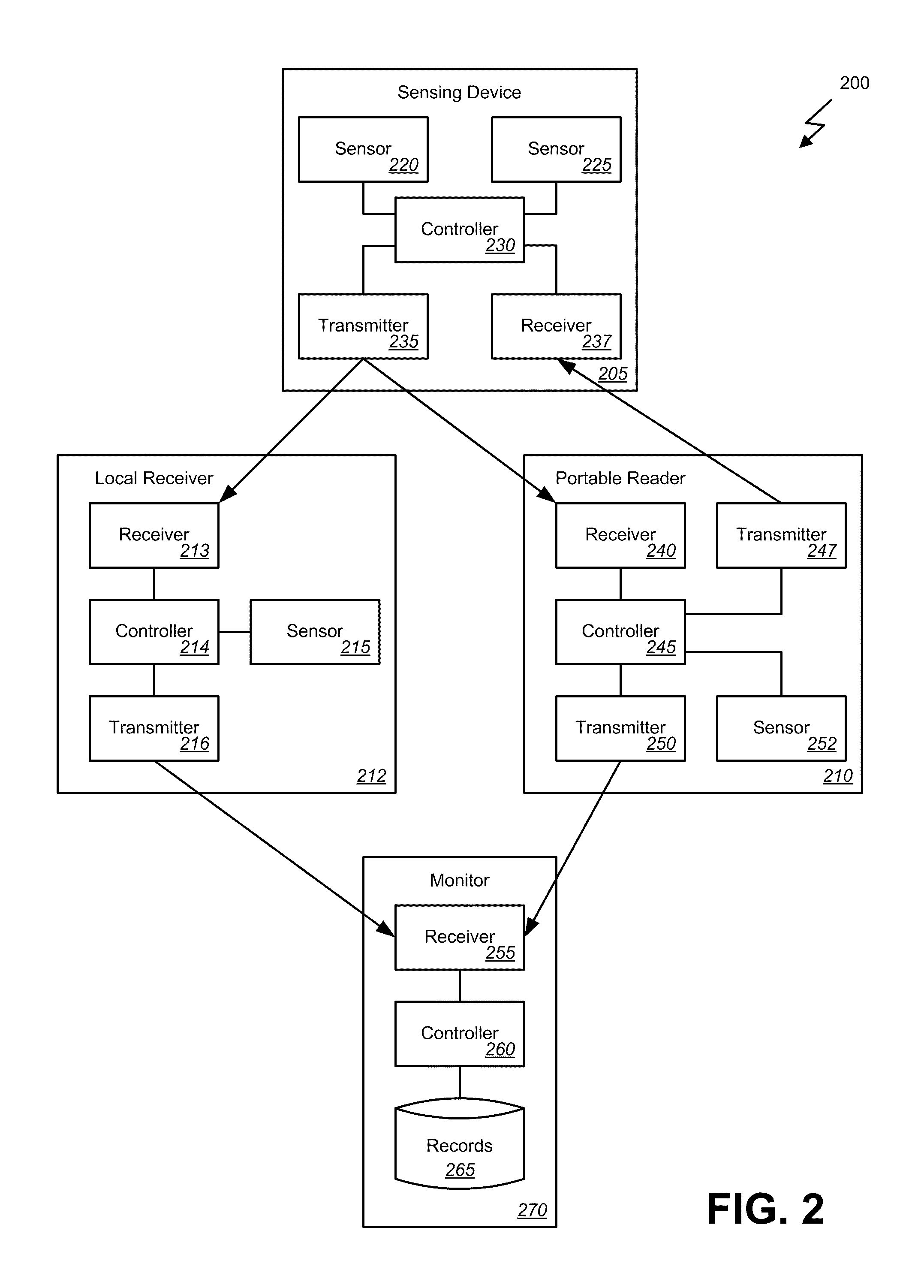
Multi-sensor patch and system
InactiveUS20110213559A1StethoscopeMeasurement arrangements for variableEngineeringHigh body temperature
Embodiments of the invention provide systems and methods for remote sensing and / or monitoring utilizing a sensing device, such as may be implemented in a patch that can be placed on or affixed to a subject, where the sensing device includes multiple sensors. For example, one embodiment of the present invention includes a wireless human temperature skin patch providing accurate measurement of human temperature from a sensing device applied to the skin and even in the presence of differing ambient temperature. In such an embodiment, the patch can include, for example, a flexible, breathable bandage or adhesive strip or pad to affix the sensing device to a patient. The sensing device can include multiple sensors such as two or more temperature sensors that can be used to accurately determine the patient's core body temperature from the measured temperature at the skin.
Owner:PRIMA TEMP
Tissue conforming microneedle array and patch for transdermal drug delivery or biological fluid collection
Microneedle arrays are provided for use on a contoured or flexible tissue surface. In one embodiment, the microneedle array includes a plurality of microneedles, each having a base portion, a tip end portion distal to the base portion, and body portion therebetween; and a flexible substrate which comprises a plurality of apertures, each of which are defined by (i) a plurality of substrate elements which are integral with the base portions of the microneedles, and (ii) at least one spring element connecting at least two of the substrate elements. The spring element may include a curved element, such as a C-shaped, U-shaped, or S-shaped element. Apertures may be defined, for example, by two substrate elements, which connected to three or four spring elements. A skin patch is provided for therapeutic or diagnostic applications, which includes the microneedle array and an adhesive material.
Owner:YUZHAKOV VADIM V
Oxygen sensor
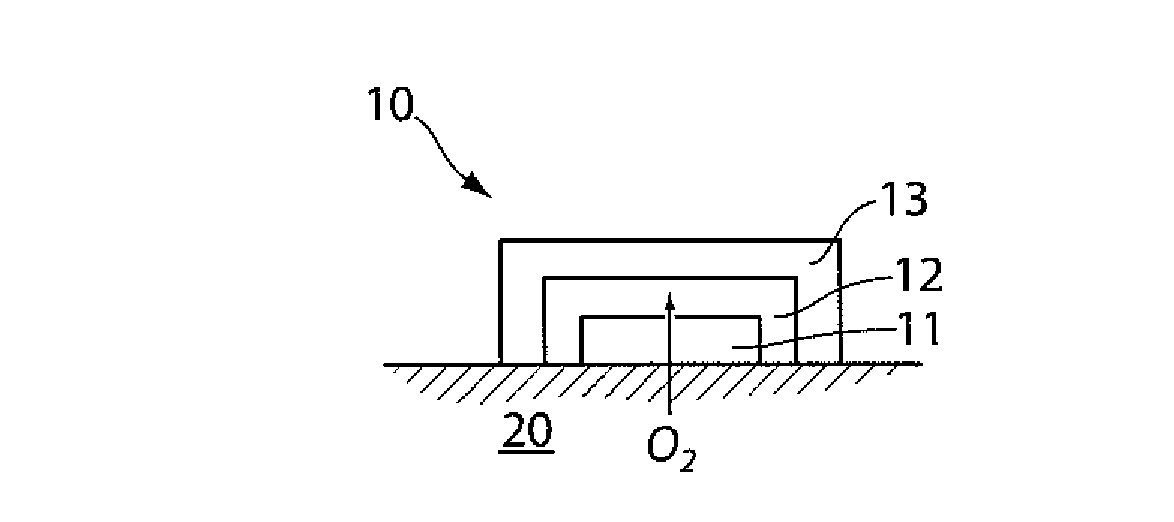
The present invention generally relates to systems and methods for determining oxygen in a sample, or in a subject. In one aspect, the present invention is generally directed to an article exhibiting a determinable feature responsive to oxygen, such as oxygen-sensitive particles. The particles may exhibit a determinable change with a change in oxygen concentration, and such particles can accordingly be used to determine oxygen. For example, in one set of embodiments, the particles may be at least partially coated with a protein, such as hemoglobin, that is able to interact with oxygen. In some cases, the protein may aggregate under certain conditions (e.g., under relatively low oxygen concentrations), and such protein aggregation may be used, for example, to cause the particles to become aggregated, which can be determined in some way. In some cases, such aggregation may be irreversible; i.e., the degree of aggregation corresponds to the most extreme oxygen concentrations that the proteins were exposed to. Such articles may be used, for example, to determine oxygen within a sample, or within a subject, such as a human subject. For instance, the article may be formed as a skin patch, or administered to the skin of a subject, e.g., on the surface of the skin, within the dermis or epidermis, etc., to determine oxygen within the subject.
Owner:SEVENTH SENSE BIOSYST
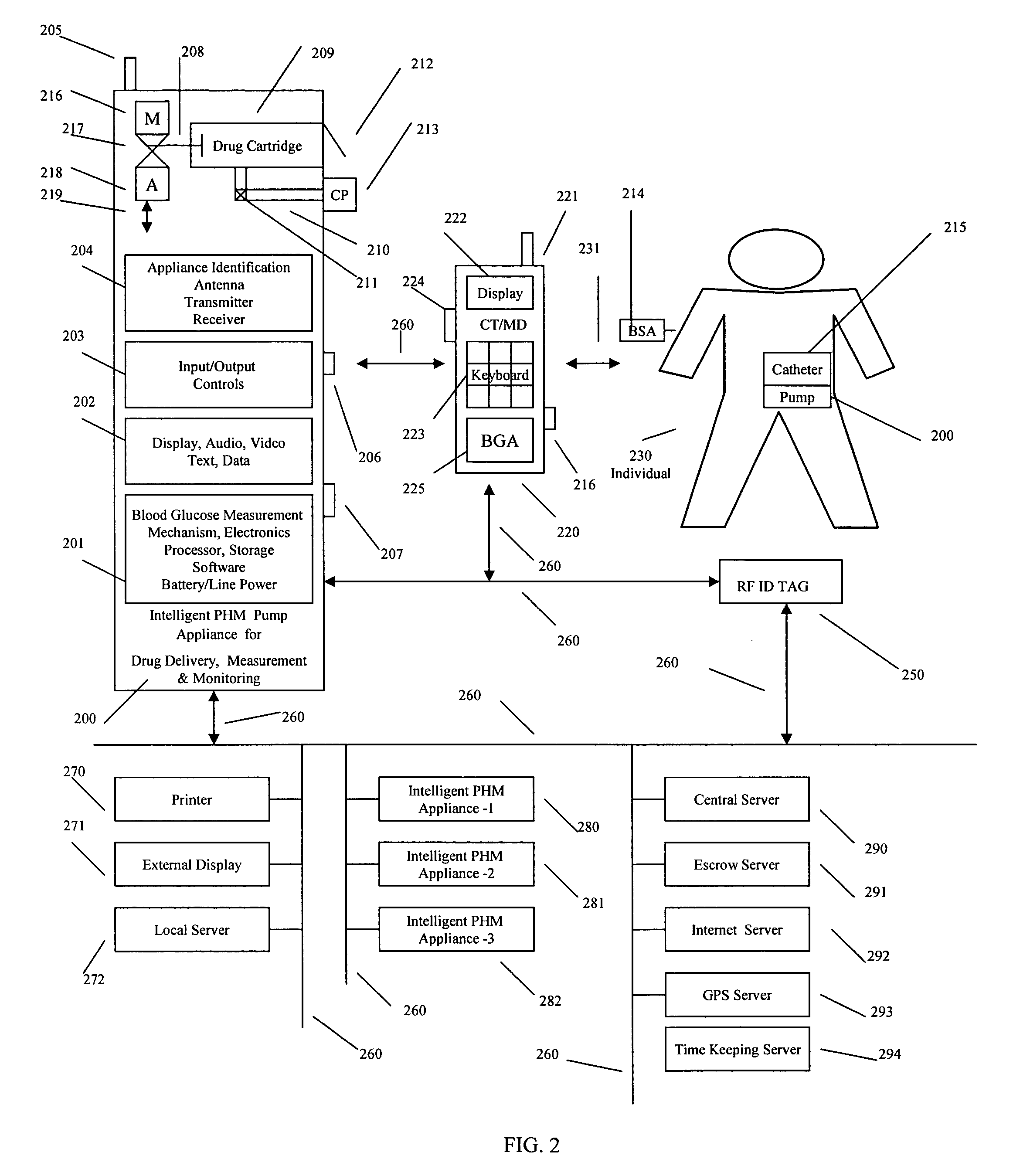
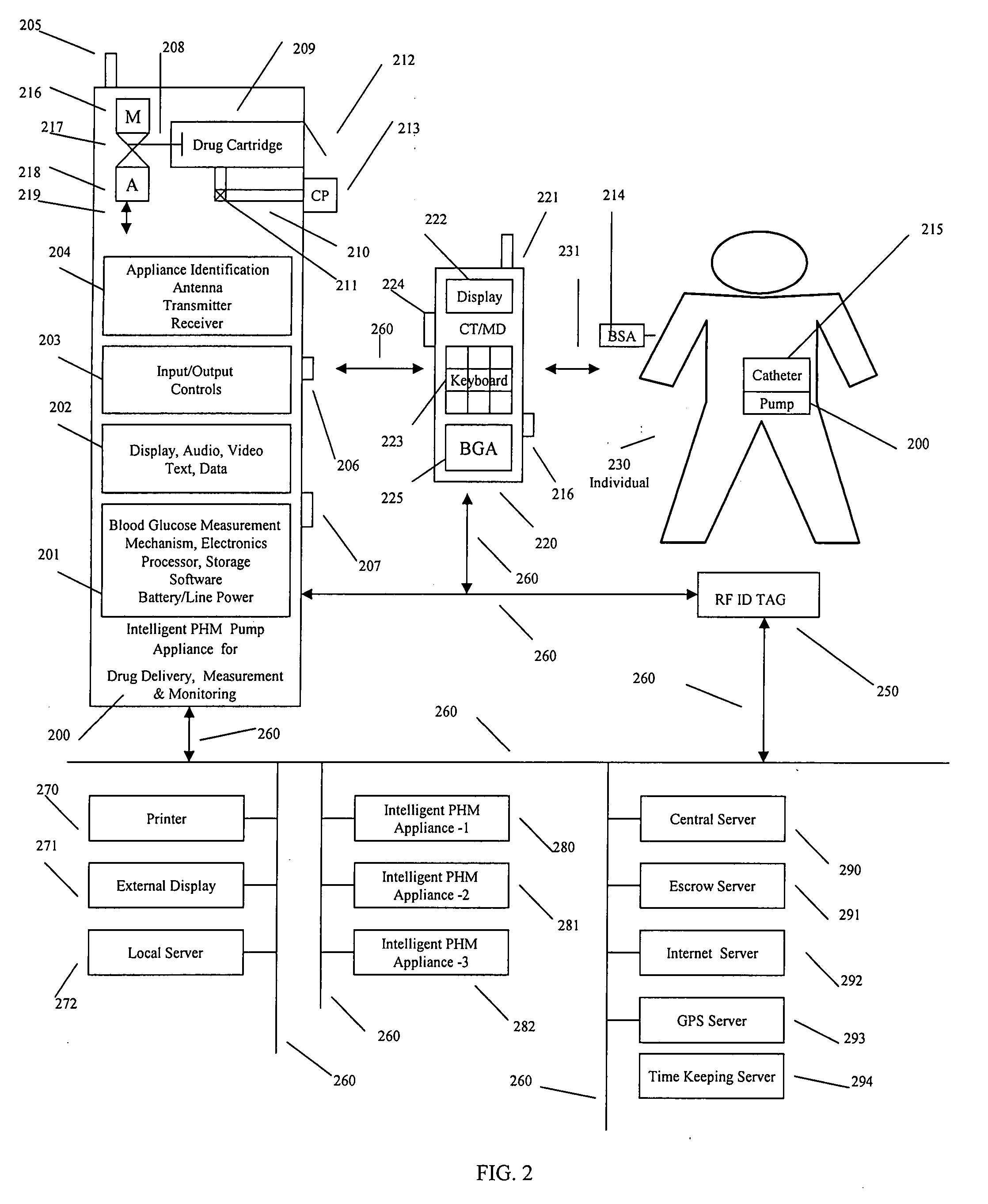
Intelligent drug delivery appliance
InactiveUS20120190955A1Precise positioningPointing accuratelyPressure infusionDiagnostic recording/measuringDiseaseDiabetes mellitus
A system, method and apparatus for real time measurement and monitoring of various personal health parameters including controlled delivery of drugs / medications by intelligent pump appliances, intelligent inhalation appliances and intelligent skin patch appliances used in a standalone manner or in a wired or wireless networked configuration, in conjunction with various peripheral devices, other intelligent appliances, servers, RF ID Tags and stationary / mobile devices. The intelligent appliances relate to the measurement, monitoring and delivery of insulin / other drugs for the treatment of diabetes and other diseases. The method also additionally includes the application of intelligent appliances for pain management including visualization of organs and body locations exhibiting pain.
Owner:IP HLDG LLC
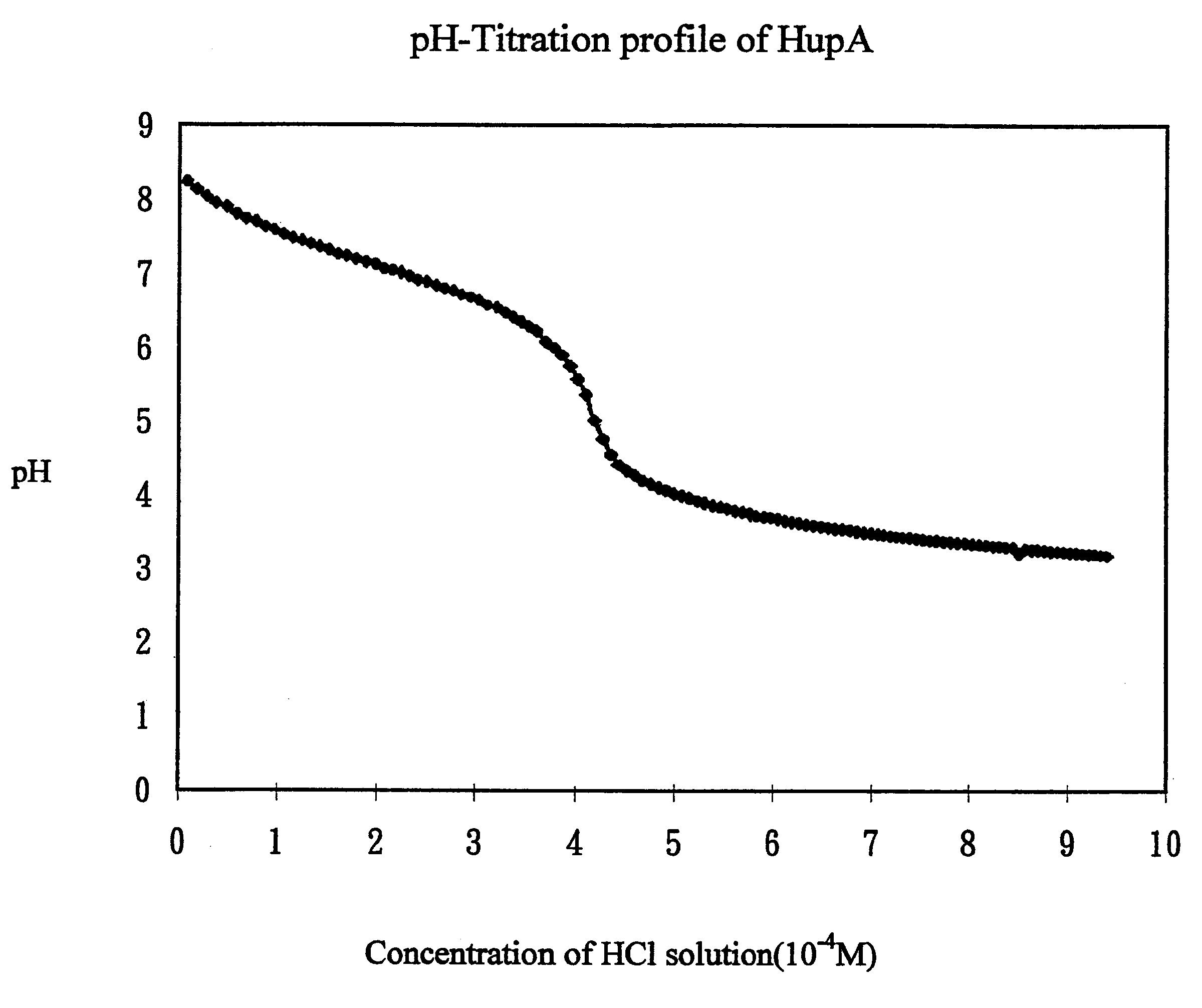
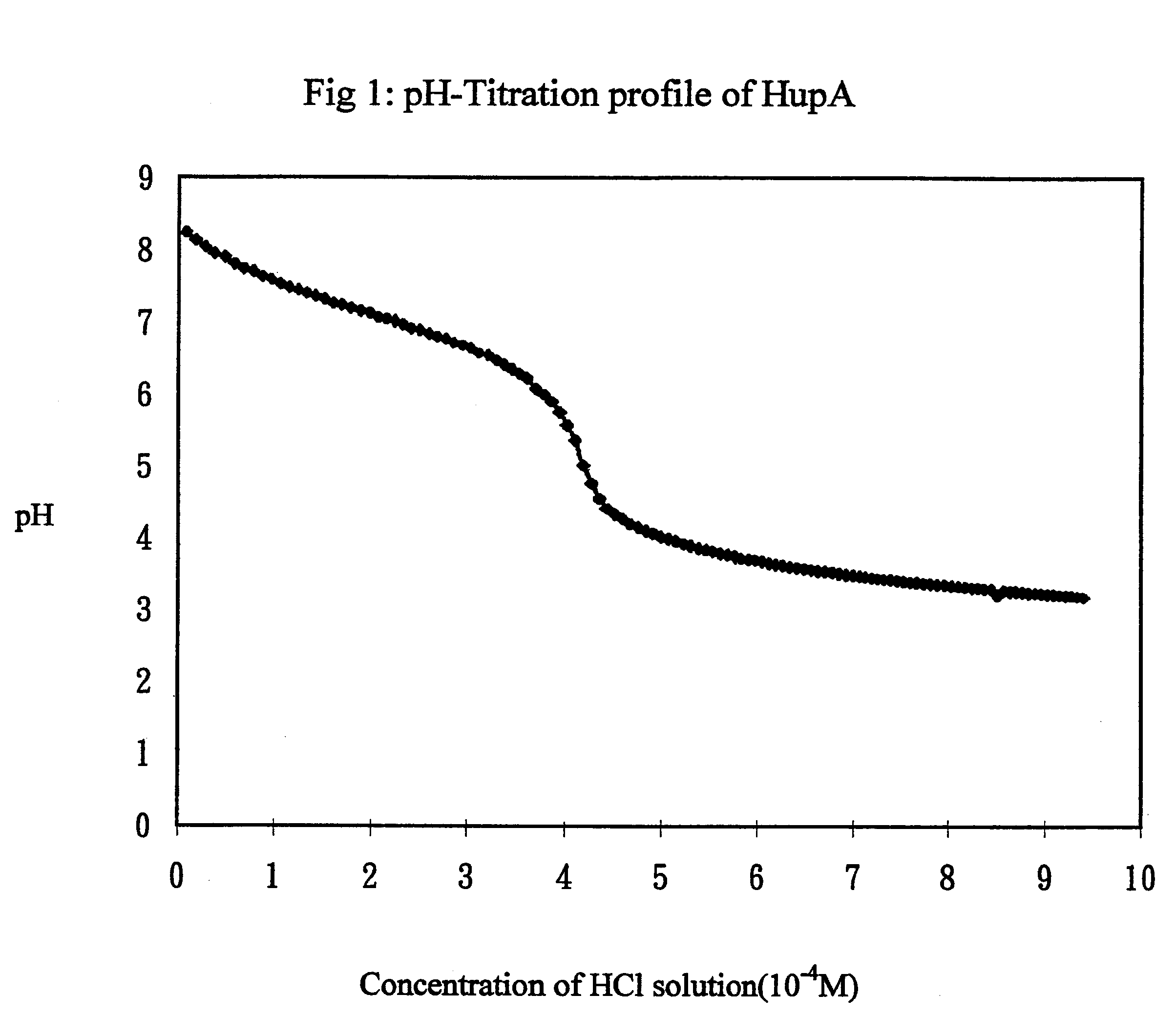
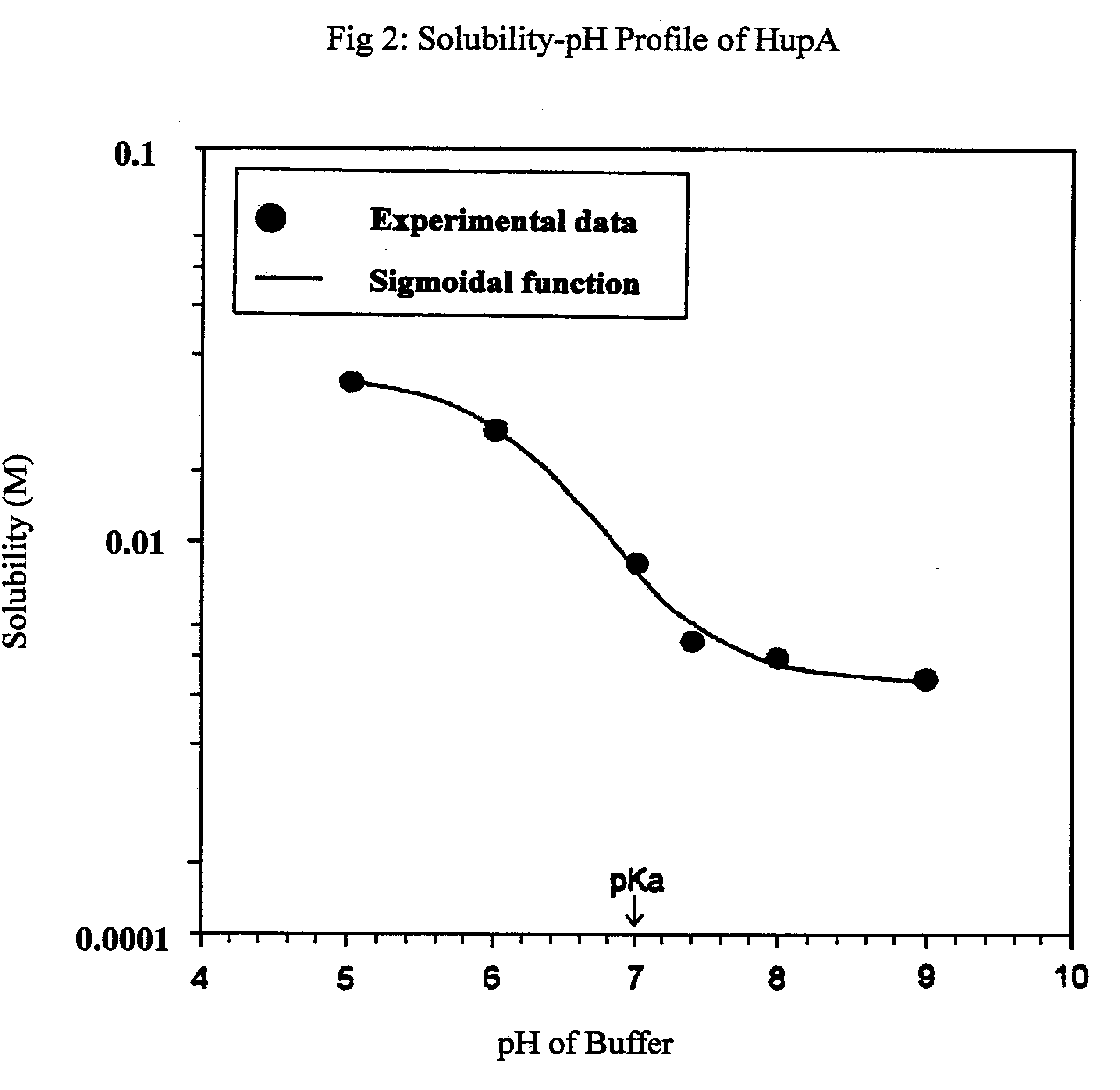
Transdermal rate-controlled delivery of Huperzine A for treatment of alzheimer's disease
This invention relates to a novel transdermal drug delivery system whereby Huperzine A ("Hup A"), a naturally occurred Acetylcholine esterase inhibitor traditionally used to alleviate memory problem, is formulated for transdermal administration suitable for the treatment of Alzheimer's Disease ("AD") to increase the efficacy and convenience for outpatient care of AD patients. A controlled-release skin patch designed for once-a-week application of Hup A is provided for easy AD medication according to the invention.
Owner:SAGITTARIUS LIFE SCI
Method of treating eosinophilic esophagitis
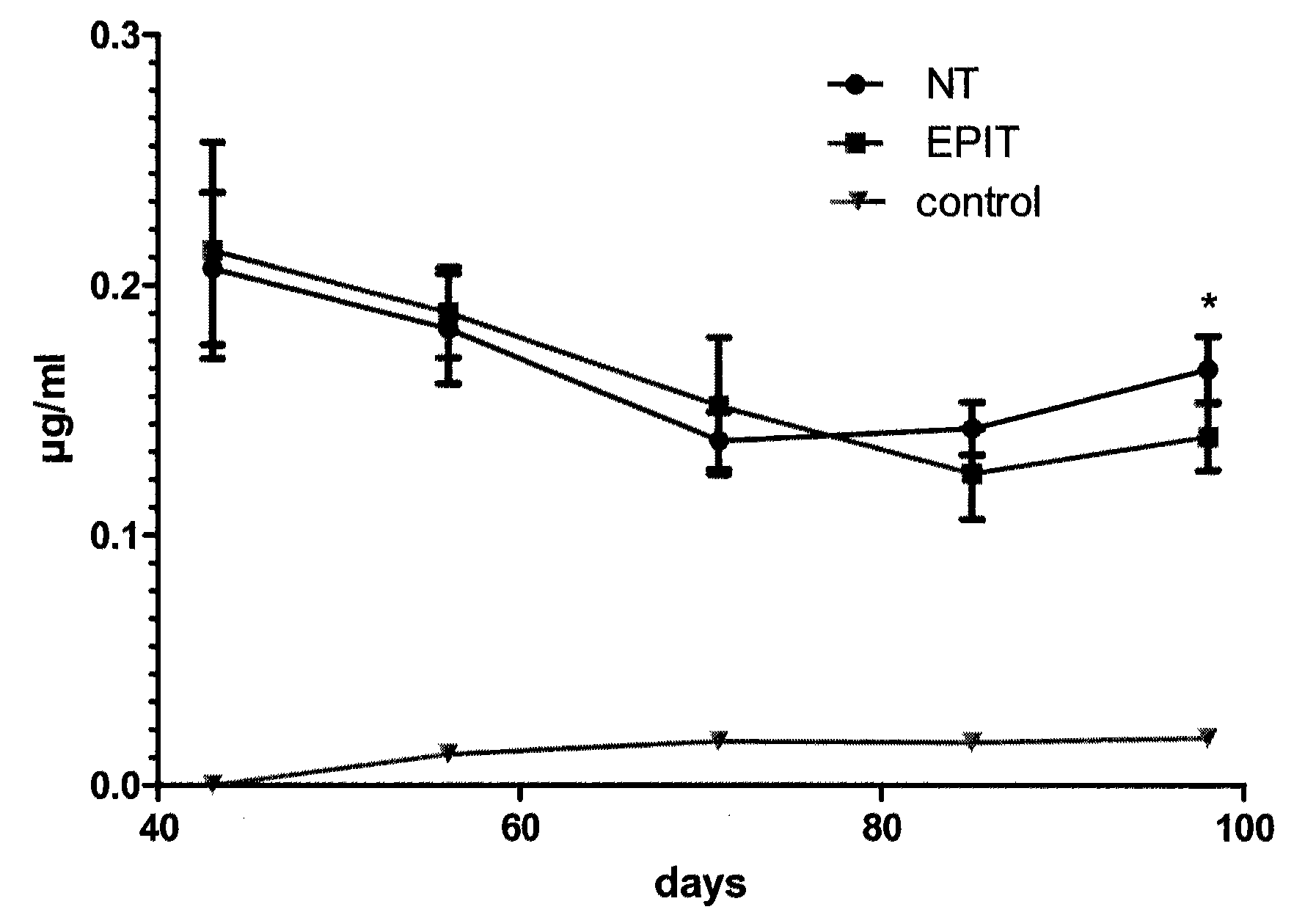
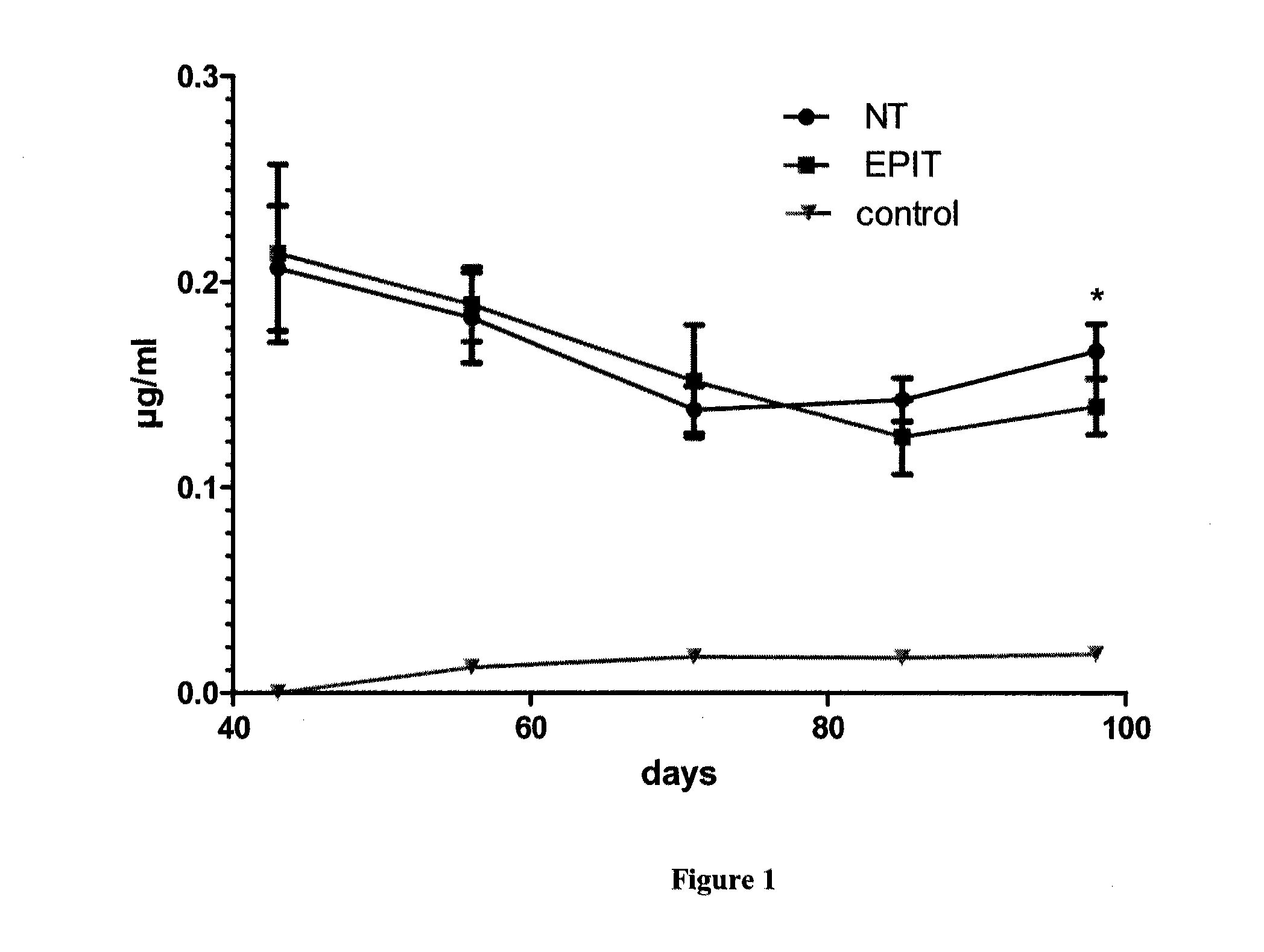
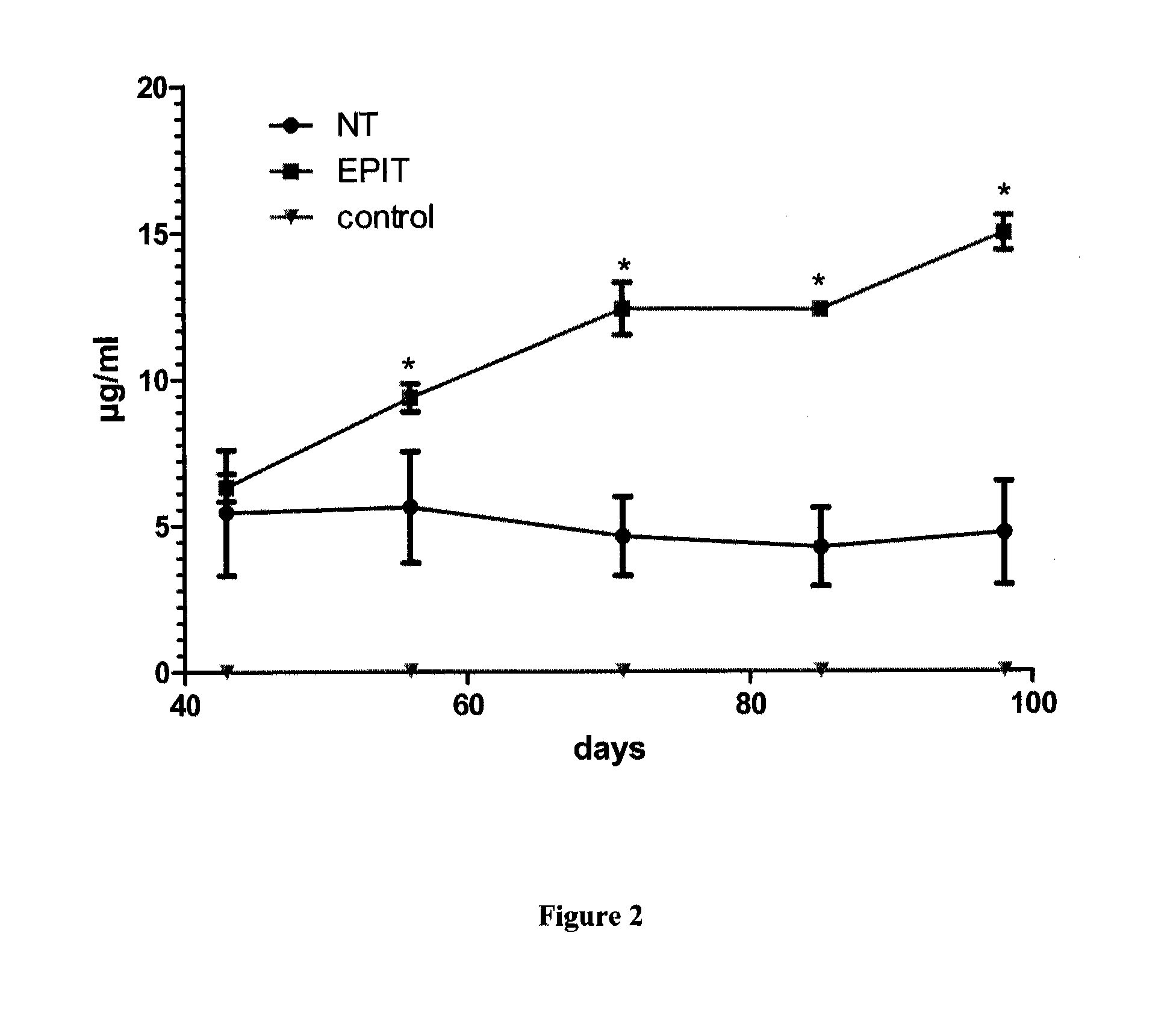
ActiveUS20120207815A1Reduce penetrationDecrease of the other histological patterns of EEDigestive systemAllergen ingredientsDermatologySkin exposure
The present invention relates to the treatment of EE. More specifically, the invention relates to a new method of treating EE through the epicutaneous route. In particular, the method of the invention comprises applying to the skin of the subject a skin patch device, comprising a composition, under conditions allowing a contact between said composition and the skin. The present invention also relates to the skin patch device and to a use of the skin patch device in the manufacture of a composition for treating eosinophilic esophagitis in a subject.
Owner:DBV TECH INC
Highly conformable adhesive device for compound, moving or variable forms
An adhesive device for compound or moving forms includes a pliable backing layer or shell. A cloth scrim including interlocking fibers oriented to provides elasticity in at least one direction. The interlocking fibers are straight and are arranged in a bias weave or the interlocking fibers are knitted or woven circular rings, and provide elasticity in multiple directions. The cloth scrim is laminated to the pliable backing layer or shell. An adhesive coating is applied to the cloth scrim for adhering the adhesive device to the compound or moving forms where the elasticity of the cloth scrim allows the adhesive device to conform to the compound or moving forms while minimizing wrinkling or puckering of the adhesive device. The edges of the cloth scrim laminated to the shell or pliable backing layer are pinked to provide increased linear contact at the edges thereby reducing de-lamination of the adhesive device from the surfaces of the compound or moving forms, and further minimizing wrinkling or puckering of the adhesive device. Such characteristics are particularly desirable in applications where intimate and very close contact between the adhesive device and the compound or moving form is crucial to the performance of the device for example in Transdermal Drug Delivery Systems (Skin Patches)
Owner:FRANCIS MICHAEL DAVID
Analyte sensor receiver apparatus and methods
InactiveUS20180153450A1Enhances and enables performanceReduces wearer weightEndoradiosondesCatheterGlucose sensorsAnalyte
Receiver apparatus for use with an analyte sensor, and methods of operation and manufacturing. In one embodiment, the analyte sensor is an implanted / implantable blood glucose sensor, including oxygen-based detector elements. The receiver apparatus is a wireless-enabled small form-factor device with limited functionality that can be easily worn or kept with the user on a continual basis, thereby obviating the need for a more fully featured receiver or smartphone for extended periods of time (e.g., one week). The exemplary oxygen based analyte sensor, with high degree of stability over time, enables the user to divorce themselves from the more fully functioned receiver or smartphone, since no external calibration of the sensor is required during the extended period. In one variant, the device is a lightweight wristband. Other variants include e.g., pendants, finger-worn rings, arm or head bands, skin patches, and even dental, subcutaneous, or prosthetic implants.
Owner:GLYSENS
Devices, methods, and kits for non-invasive glucose measurement
InactiveUS7725149B2Minimize impactMicrobiological testing/measurementSurgeryMeasurement deviceDisplay device
Described are devices, methods, and kits for non-invasively measuring glucose. In general, the devices comprise skin patches for placement on a skin surface and measurement devices for measuring glucose collected in the patches. The patches may include an adhesive material, a collection layer, an interface layer, and a sweat-permeable membrane. The sweat-permeable membrane is configured to act as a barrier to epidermal contaminants and glucose brought to the skin surface via diffusion. In this way, non-correlatable skin surface glucose will not be measured. The patches may further include components to induce a local sweat response. The measurement device typically includes a display, a processor, and a measurement mechanism. The methods typically include the steps of wiping the skin surface with a wipe containing at least one solvent for removing glucose, placing a patch on a skin surface, and measuring glucose collected in the patch. Kits comprising the patch and measurement device are also described.
Owner:VIVOMEDICAL INC
Skin-attachable drug injection device with detachment sensor
ActiveUS20170259015A1Reduce disruptionReduce flow rateAutomatic syringesMedical devicesDrug injectionInjection site
A skin-patch type large volume medicament injection device (1) comprising an adhesive surface (21 / 23) for attachment to the skin and a plurality of contact sensors (24) configured to output a signal representing a degree of detachment of the device from the injection site, and an alarm and control function which outputs an alert to the user based on the signal received from the sensors and stops the injection in case the degree of detachment is too large to guarantee further save injection.
Owner:SANOFI SA
Film base material for adhesive skin patch and adhesive skin patch
InactiveUS20050169975A1Change physical propertiesFailure in characteristicAdhesive dressingsAbsorbent padsFilm basePolypropylene glycol
To have acceptable moisture permeability and enable prevention of deformation due to swelling, the film base material for an adhesive skin patch includes an ether-based urethane resin obtained from at least one member selected from the group consisting of polyoxytetramethylene glycol, butanediol, polyethylene glycol, and polypropylene glycol as a diol component, and methylene diphenyl-diisocyanate as an isocyanate component. The film base material for an adhesive skin patch has a moisture permeability of preferably 800 to 4,000 g / m2·24 hrs. The adhesive skin patch can be produced by forming a pressure-sensitive adhesive layer on one side of the film base material for an adhesive skin patch.
Owner:NITTO DENKO CORP
Pain tracking and management device
InactiveUS20120190936A1Pointing accuratelyPhysical therapies and activitiesElectrotherapyDiabetes mellitusDisease
A system, method and apparatus for real time measurement and monitoring of various personal health parameters including controlled delivery of drugs / medications by intelligent pump appliances, intelligent inhalation appliances and intelligent skin patch appliances used in a standalone manner or in a wired or wireless networked configuration, in conjunction with various peripheral devices, other intelligent appliances, servers, RF ID Tags and stationary / mobile devices. The intelligent appliances relate to the measurement, monitoring and delivery of insulin / other drugs for the treatment of diabetes and other diseases. The method also additionally includes the application of intelligent appliances for pain management including visualization of organs and body locations exhibiting pain.
Owner:IP HLDG LLC
Topical Copper Ion Treatments and Methods of Treatment Using Topical Copper Ion Treatments in the Dermatological Areas of the Body
ActiveUS20140271797A1Good lookingImprove skin appearanceSuture equipmentsBiocideDiseaseWound dressing
Owner:CDA RES GROUP
Skin Patch Sheet, Use Thereof, and Method for Attaching Skin Patch Sheet
Provided are a skin patch sheet including a carrier sheet, an adhesive patch and a release liner laminated in this order, in which (a) the adhesive patch includes two layers, namely, a backing and an adhesive layer having the same shape; (b) the carrier sheet is adjacent to the backing of the adhesive patch; (c) the skin patch sheet includes a grip section and a body section; (d) in the body section or the body section and grip section, the area of the release liner is larger than the area of the adhesive patch; (e) the boundary between the grip section and the body section is a slit line formed by a slit; and (f) the slit extends from the side of the release liner, penetrating into the adhesive patch, up to a depth in the range of greater than or equal to 20% and less than 50% of the thickness on the adhesive patch side of the carrier sheet; a skin patch sheet in which plural sheets of adhesive patches on which carrier sheets are laminated are formed in a parallel arrangement on a release liner; use of the skin patch sheet by attaching the skin patch sheet to the skin surface in order to prevent direct scratching of an affected site of the skin, or by attaching the skin patch sheet to the face for a lift-up effect; and a method for attaching the skin patch sheet.
Owner:NICHIBAN CO LTD
Skin-patch type infusion pump comprising a resonant buzzer
InactiveUS8773257B2Low energy consumption behaviorCheap methodMedical devicesPressure infusionEngineeringBiological activation
Disclosed are methods and devices that include a therapeutic fluid dispensing device (10) to deliver a therapeutic fluid into a body of a patient. The device includes a controller to control one or more of fluid delivery operations and notification operations, at least one auditory notifier (800) to produce one or more acoustic signals in response to application of one or more activation signals by the controller and a plurality of electrical contacts coupled to the at least one auditory notifier to enable the application of the one or more activation signals to the at least one auditory notifier. The device also includes at least one housing retaining the at least one auditory notifier therein, the at least one housing being structured to resonate at least one of the one or more acoustic signals produced by the at least one auditory notifier in response to application of at least one of the one or more activation signals.
Owner:ROCHE DIABETES CARE INC
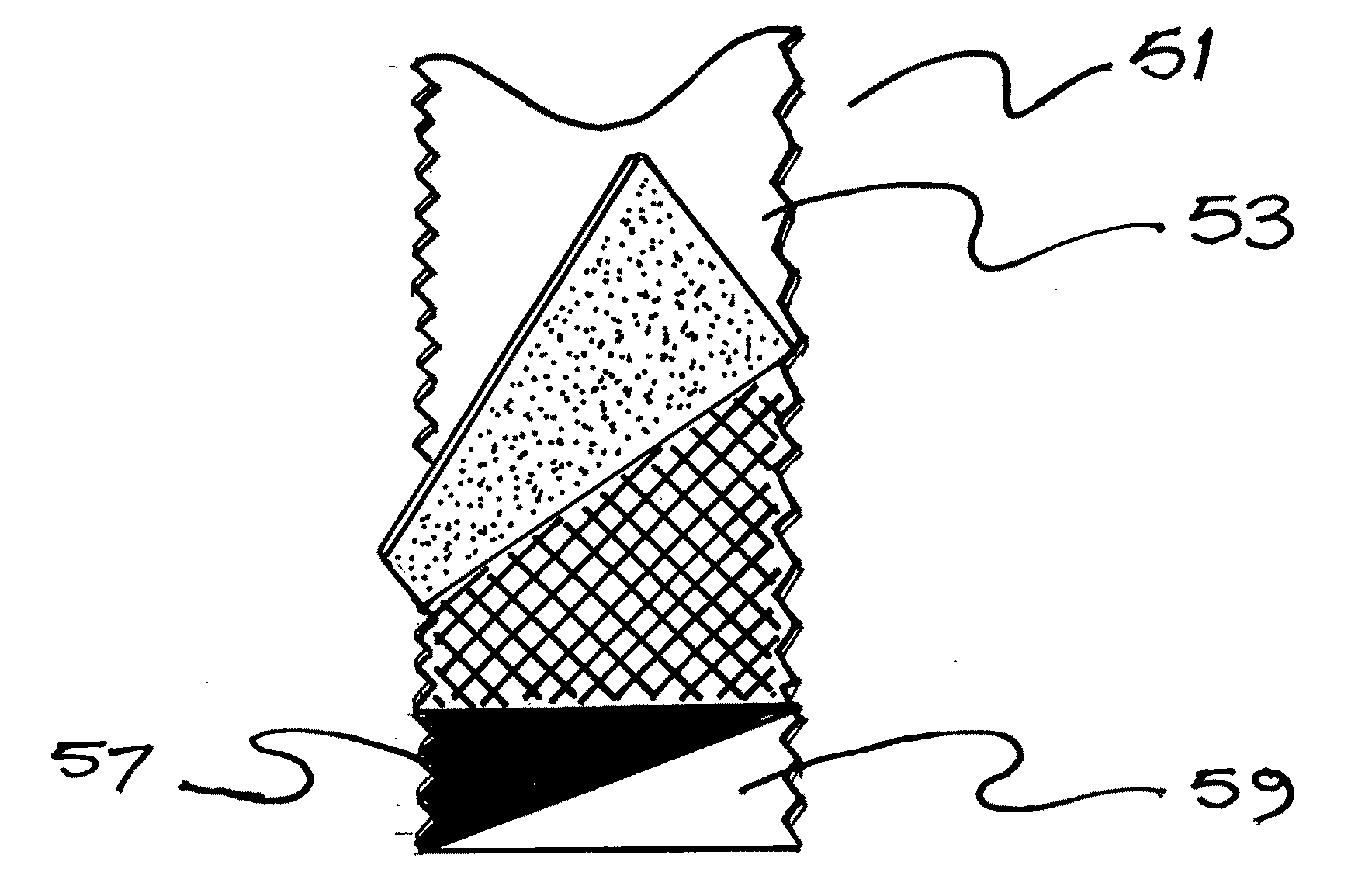
Wrinkle reducing skin patch, process of manufacture and useful articles thereof
ActiveUS20090234382A1Good effectImprove permeabilityHead bandagesCosmetic preparationsWrinkle skinTopical surface
This invention relates to a novel method of reducing the appearance of rhytides (“facial wrinkles”) and / or infra-orbital shadows (“dark circles”) under the eyes, and striae (“stretch marks”), or lentigo senilis (“age spots”), and hyper / hypo pigmentation on other anatomical areas. Particularly, this invention relates to a topical patch worn on the skin that in a preferred embodiment incorporates two distinct layers, each providing useful features and together providing a novel article and method of improving skin cosmesis. Ease of use, patient comfort and cost effectiveness are provided. The patch is applied to the affected area and preferably worn at least several hours and more preferably worn overnight. The product increases the hydration level of the skin over which it is applied. There is a slight increase to the volume of the local superficial tissue, which serves to tighten the overlying skin and reduce the size and quantity of fine wrinkles at least temporarily. Various active ingredients, such as peptides, vitamin compounds, antioxidants, growth factors, glycolic or alphahydroxy acids, etc., may be incorporated into the present invention thereby providing an enhanced effect or other perceived advantage in the market.
Owner:BIO MED SCI
Chitosan vehicle and method for making same
InactiveUS20100086613A1Powder deliveryPharmaceutical non-active ingredientsChitosan nanoparticlesProtein-protein complex
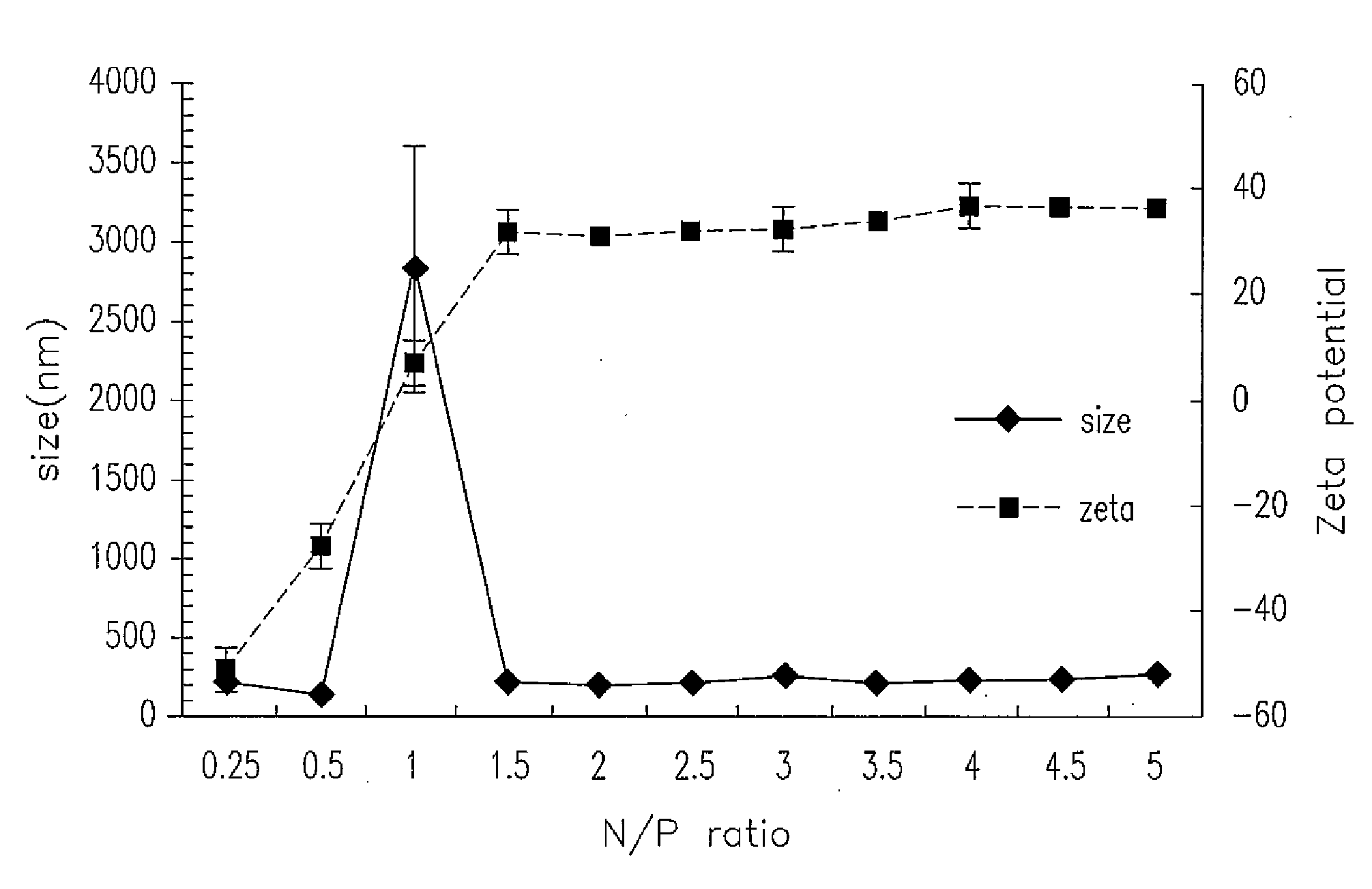
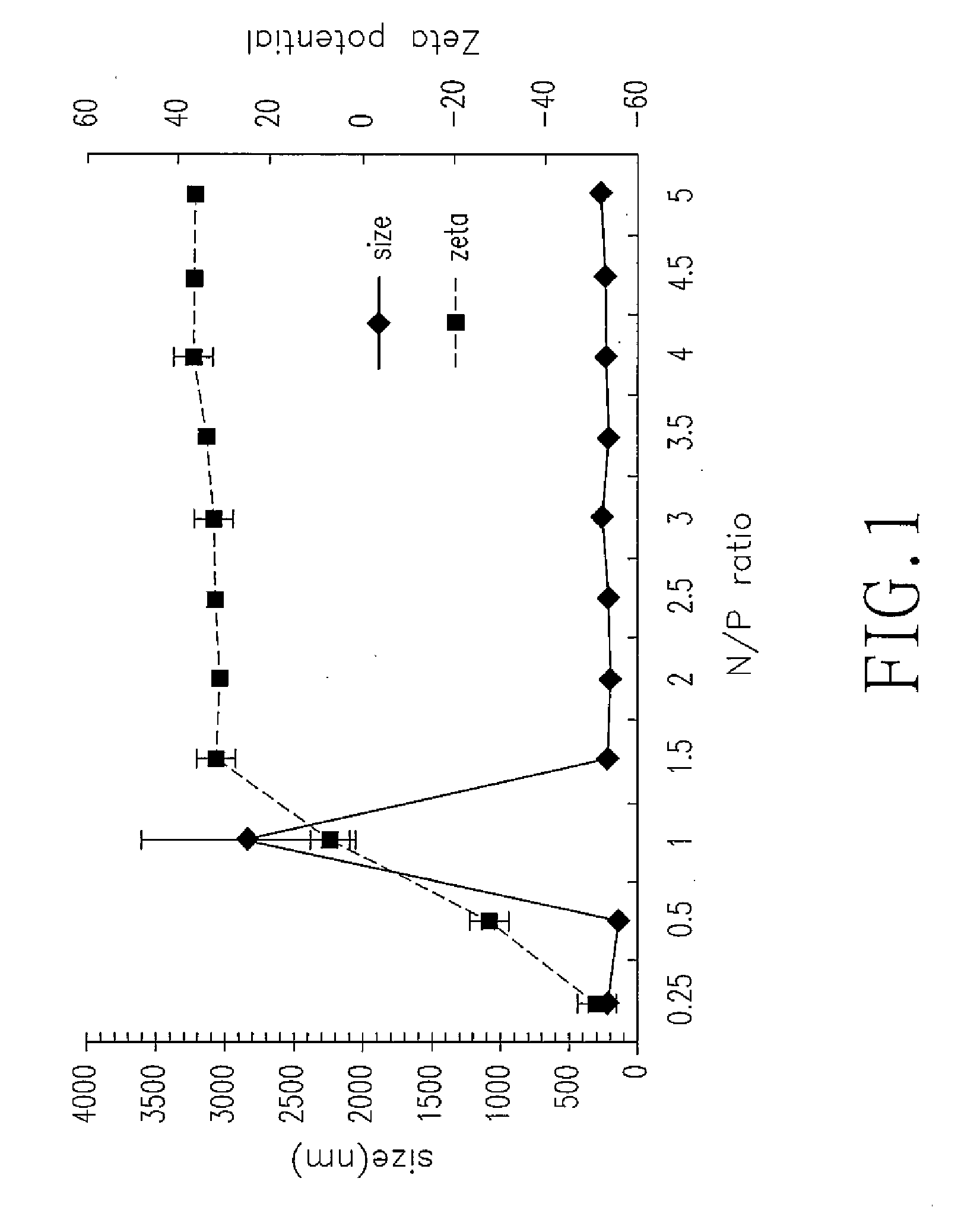
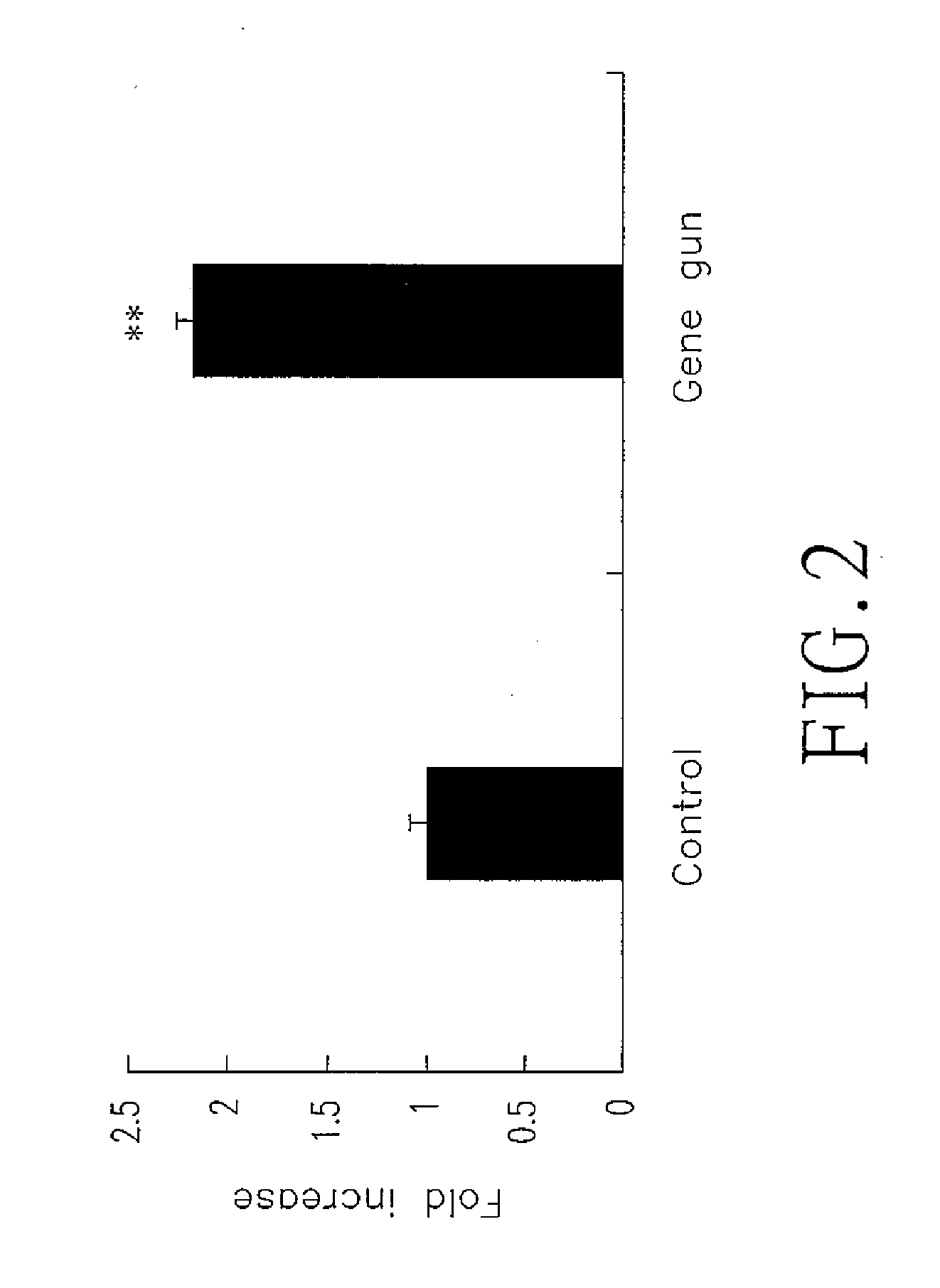
The invention relates to chitosan (CS) vehicles with chitosan nanoparticles, as well as methods for making such chitosan vehicles and for using them to carry a DNA or proteins by forming CS-DNA or CS-protein complexes. The present invention also relates to CS-DNA or CS-protein complexes being useful for transdermal delivery of DNA or protein with a low-pressure gene gun. In another aspect, the present invention also relates to CS-DNA or CS-protein complexes being useful for transcutaneous delivery of a DNA or protein with a skin patch. Further aspects of the present invention relate to methods for making CS-DNA or CS-protein complexes and for using them for diagnostic, therapeutic and biological industrial applications.
Owner:WU CHANG JER +3
Film base material for adhesive skin patch and adhesive skin patch
The present invention relates to a film base material for an adhesive skin patch, which includes an elastomer film unevenly having unevenness on at least one surface thereof, in which, in one unevenness in which the distance perpendicular to the surface of the elastomer film between the top of the unevenness and the bottom thereof is the longest among the plurality of unevenness, the distance is within the range of from 1 to 5 μm; in another unevenness in which the distance perpendicular to the surface of the elastomer film between the top of the unevenness and the bottom thereof is the shortest among the plurality of unevenness, the distance is within the range of from 0.1 to 0.9 μm; and the 10-point average roughness of the surface is within the range of from 0.5 μm to 3 μm, and which is decreased in glossy texture on the surface thereof and inconspicuous when it is applied to the skin or the like; and the adhesive skin patch including the film base material.
Owner:NITTO DENKO CORP
Screening test and procedure using skin patches
Marker compounds indicative of variations in physiological conditions, genetic defects or disease states have been found to be present in apocrine sweat and, in many conditions, these markers are present in apocrine sweat in elevated levels when compared to blood. These markers are collected and / or detected in apocrine sweat and used as an indicator that a disease state or particular physiological condition exists. A skin patch, wipe or electronic detector for vapors released from apocrine sweat are used to indicate the existence of a disease state or changing physiological condition. The patch or wipe can include in monoclonal antibodies or other chemical compounds that can indicate the presence of specific markers. This may include indicators that render visible, the existence of a reaction between the monoclonal antibody or chemical compound and the marker compound to indicate the existence of the condition being tested for. Various different disease states can be detected including, but not limited to heart disease, cancer, autoimmune disease, viral or bacterial disease, renal disease, drug intoxication and other systemic illnesses. Fertility for conception and histocompatibility for organ transplantation of individuals should also be detectable.
Owner:HERMETIC DIAGNOSTICS
Topical Copper Ion Treatments and Methods of Making Topical Copper Ion Treatments for Use in Various Anatomical Areas of the Body
A topical copper ion treatment in basic form comprises a copper ion-containing solution composed of a biocompatible solution containing copper ions obtained by leaching of the copper ions from copper metal into the solution. The copper ion-containing solution can be combined with various carriers to form various forms of the copper ion treatment including creams, gels, lotions, foams, pastes, tampons, solutions, suppositories, body wipes, wound dressings, skin patches, and suture material. A method of making the copper ion-containing solution involves placing solid copper metal in a quantity of a biocompatible solution and maintaining the solution at a specified temperature for a predetermined period of time during which copper ions leach from the copper metal into the solution, and thereafter separating the solution from the solid copper metal.
Owner:CDA RES GROUP
Methods of treatment using topical copper ion formulations
ActiveUS20170000823A1Inhibits biofilm formationInhibition formationCosmetic preparationsAnimal huntingWound dressingTopical treatment
Provided herein are topical formulations containing copper ions and methods of treating inflammatory, microbial, and arthritic conditions in various areas of the body using such formulations. Methods of treating osteoarthritis using topical copper ion treatments are provided. Methods of treating and preventing microbial infections using copper ion treatments are further provided, including methods of preventing biofilm. A topical treatment in its basic form comprises a biocompatible copper ion solution or suspension obtained by leaching of the copper ions from copper metal. The copper ion solution or suspension is combined with various carriers to form the copper ion treatment including creams, gels, lotions, foams, pastes, tampons, solutions, suppositories, body wipes, wound dressings, skin patches, and suture material. Methods of making the copper ion solution or suspension from solid copper metal in a biocompatible solution are also provided.
Owner:CDA RES GROUP
Topical copper ion treatments and methods of treatment using topical copper ion treatments in the dermatological areas of the body
ActiveUS10398733B2Good lookingImprove skin appearanceSuture equipmentsInorganic active ingredientsDiseaseWound dressing
Copper ion treatments for dermatological areas of the body include solutions, creams, lotions, gels, foams, wound dressings, skin patches and suture material, each containing copper ions that bring about therapeutic effects when the copper ion treatments are applied to dermatological tissue. Methods of treating dermatological areas of the body include treatments for use on the skin and nails to treat conditions including disease, infection, inflammation, damaged or injured tissue, tissue needing to be sutured, rashes and other undesirable dermatological conditions.
Owner:CDA RES GROUP
Method of treating eczema
InactiveUS20120064144A1Efficient immunotherapyReducing skin reactivityPeptide/protein ingredientsAllergen ingredientsDermatologySkin patch
The present invention relates to the treatment of eczema. More specifically, the invention relates to a new method of treating eczema through the epicutaneous route. In particular, the method of the invention comprises applying to a non eczematous area of the skin of the subject a skin patch device, comprising a composition, under conditions allowing a contact between said composition and the skin. The present invention also relates to the skin patch device.
Owner:DBV TECH INC
Screening test and procedure using apocrine sweat
Marker compounds indicative of variations in physiological conditions, genetic defects or disease states have been found to be present in apocrine sweat and, in many conditions, these markers are present in apocrine sweat in elevated levels when compared to blood. These markers are collected and / or detected in apocrine sweat and used as an indicator that a disease state or particular physiological condition exists. A skin patch, wipe or other collection means is used to collect apocrine sweat. The apocrine sweat is then used to indicate the existence of a disease state or changing physiological condition by the presence of specific marker compounds. Apocrine sweat can also be collected from individuals known to have a specific disease or physiological condition and, by comparison with apocrine sweat from normal individuals, used to identify new markers indicative of the specific disease state or physiological condition.
Owner:HERMETIC DIAGNOSTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com