Orally administrable film dosage forms containing ondansetron
A technology of ondansetron and dosage forms, applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, drug combinations, etc., can solve problems such as inappropriate or convenient for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
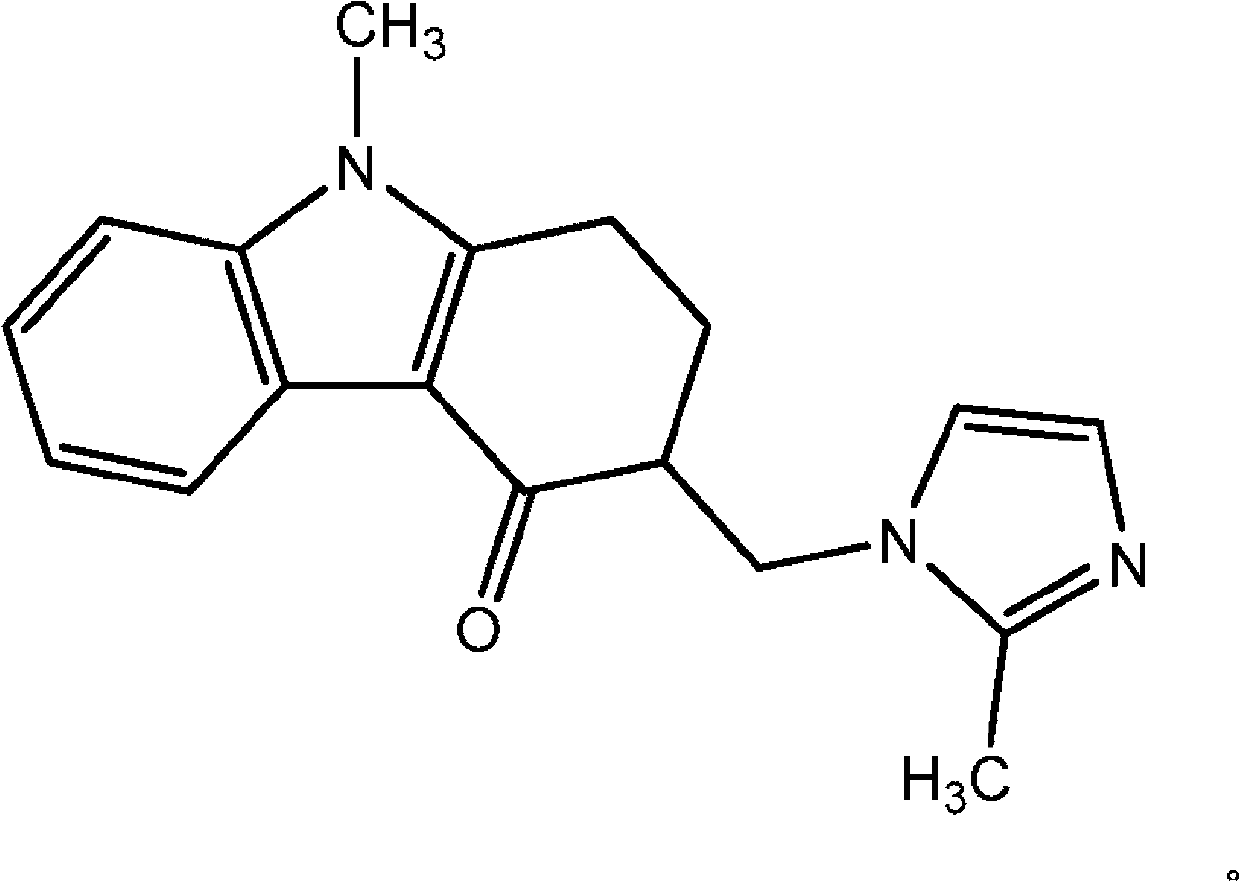
[0020] The present invention provides an orally administrable disintegrating film dosage form comprising ondansetron. The term "ondansetron" refers to ondansetron, its pharmaceutically acceptable salts, hydrates, solvates, polymorphs, complexes and prodrugs. The term "ondansetron" may refer to a racemic mixture or an enantiomer of ondansetron. The term "ondansetron" also includes any moiety that yields the active ingredient of ondansetron. In some preferred embodiments, "ondansetron" is ondansetron or the hydrochloride salt of the base of ondansetron. As used herein, the term "complex" is intended to include compounds comprising ondansetron and compounds that can be formed by any association, including by ionic bonds, by covalent bonds, by inclusion, or by any other means of forming the desired complex. ) any construct of a ligand with which it is associated.
[0021] The compositions of the present invention provide the C max and AUC values, whether the composition is adm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com