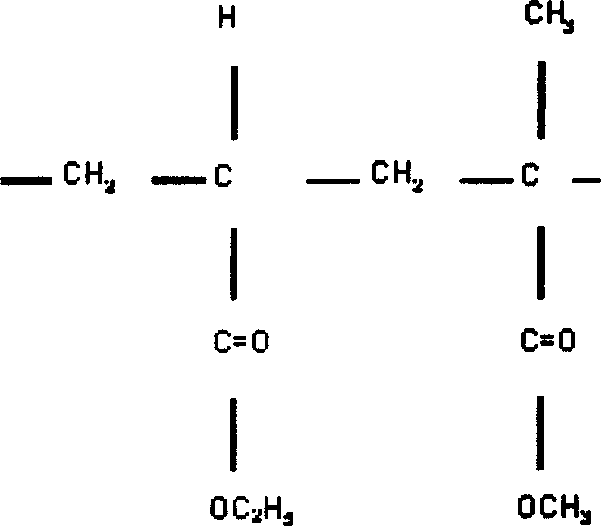
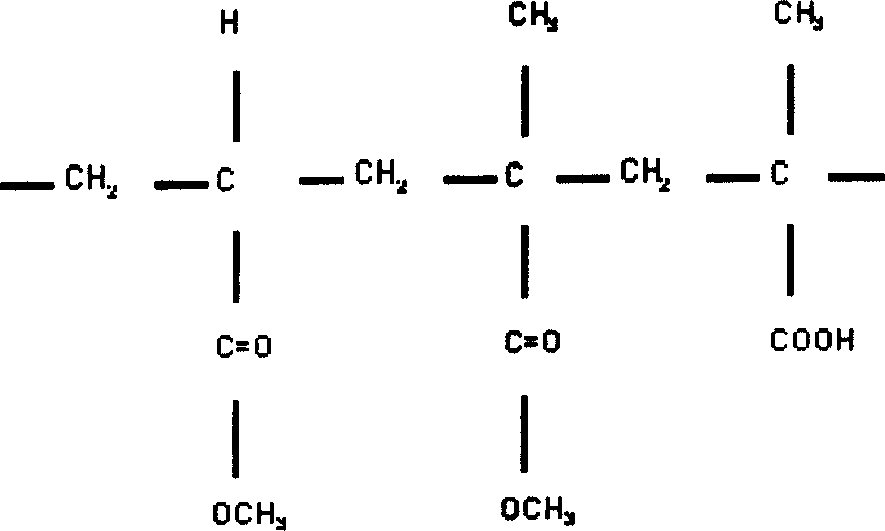
Sustained releasing talbets for radioactive and chemical therpay and preparation thereof
A technology of sustained-release pellets and sustained-release coating, which is applied in the field of sustained-release pellets and their preparation, and can solve the problems of affecting the therapeutic effect and being unable to maintain for a long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the sustained-release pellets for treating radiotherapy and chemotherapy vomiting of the present invention consists of the following steps in turn:
[0038] (1) Preparation of pill core
[0039] Method 1: Pass the raw drug through a 100-mesh sieve, mix it with microcrystalline cellulose by equal volume incremental dilution method, and then mix it with lactose. Add appropriate amount of distilled water to make a soft material, make pellets on an extrusion spheronizer, dry at 55°C for 3 hours, and sieve 18-24 mesh pellets for coating.
[0040] Method Two:
[0041] (a) Preparation of drug-containing layer coating suspension
[0042] (1) Preparation of suspension: Soak 2-10 grams of hydroxypropyl methylcellulose in 20-120 ml of 80-90 ° C distilled water for 2-8 hours, then add 0.2-10 grams of anti-sticking agent and 100-180 ml of 95% ethanol , stir evenly, and filter through a 200-mesh sieve.
[0043] (2) Preparation of drug-containing coating ...
Embodiment 1
[0062] (1) Preparation of pill core
[0063] See the preparation method 1 of the pill core.
[0064] (2) Preparation of drug-free sealing layer
[0065] Preparation of the suspension: Soak 2 g of hydroxypropyl methylcellulose in 20 ml of 80-90° C. distilled water for 2 hours, then add 0.2 g of anti-sticking agent and 100 ml of 95% ethanol, stir evenly, and filter through a 200-mesh sieve.
[0066] After 50 g of pill cores were dried at 50° C., 5 ml of suspension fluidized bed spray coating was applied to blank pellet cores, and then dried.
[0067] (3) Preparation of sustained-release coating solution
[0068] Dissolve 2.4g of porogen in water, then add 1.8g of anti-sticking agent and 16g of acrylic resin aqueous dispersion (NE30D), and add water to 60g. Stir evenly and filter through a 200-mesh sieve.
[0069] (4) Preparation of sustained-release coating layer
[0070] Use 18ml of slow-release coating solution, continue to spray coating in the ebullating bed at 23°C, and...
Embodiment 2
[0074] (1) Preparation of pill core
[0075] (1) Preparation of drug-containing layer coating suspension
[0076] (a) Preparation of suspension: Soak 2 g of hydroxypropyl methylcellulose in 20 ml of 80-90° C. distilled water for 2 hours, then add 0.2 g of anti-sticking agent and 100 ml of 95% ethanol, stir evenly, and filter through a 200-mesh sieve.
[0077] (b) Preparation of drug-containing coating suspension: add 4.5 g of ondansetron to 55 ml of 95% ethanol to dissolve, then add 6.0 ml of the suspension, and stir evenly.
[0078] (2) Preparation of drug-containing sealing layer
[0079] Adopt the model that NP PHARM company produces to be the blank ball core 50 grams of PF008 after drying, 63ml drug-containing layer coating suspension is spray-coated on the blank ball core, then dry again.
[0080] (2) Preparation of drug-free sealing layer
[0081] Containing 50 g of pill cores, after drying, 63 ml of suspension fluidized bed spray coating is applied to blank ball core...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com