System and method for intranasal administration of lorazepam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
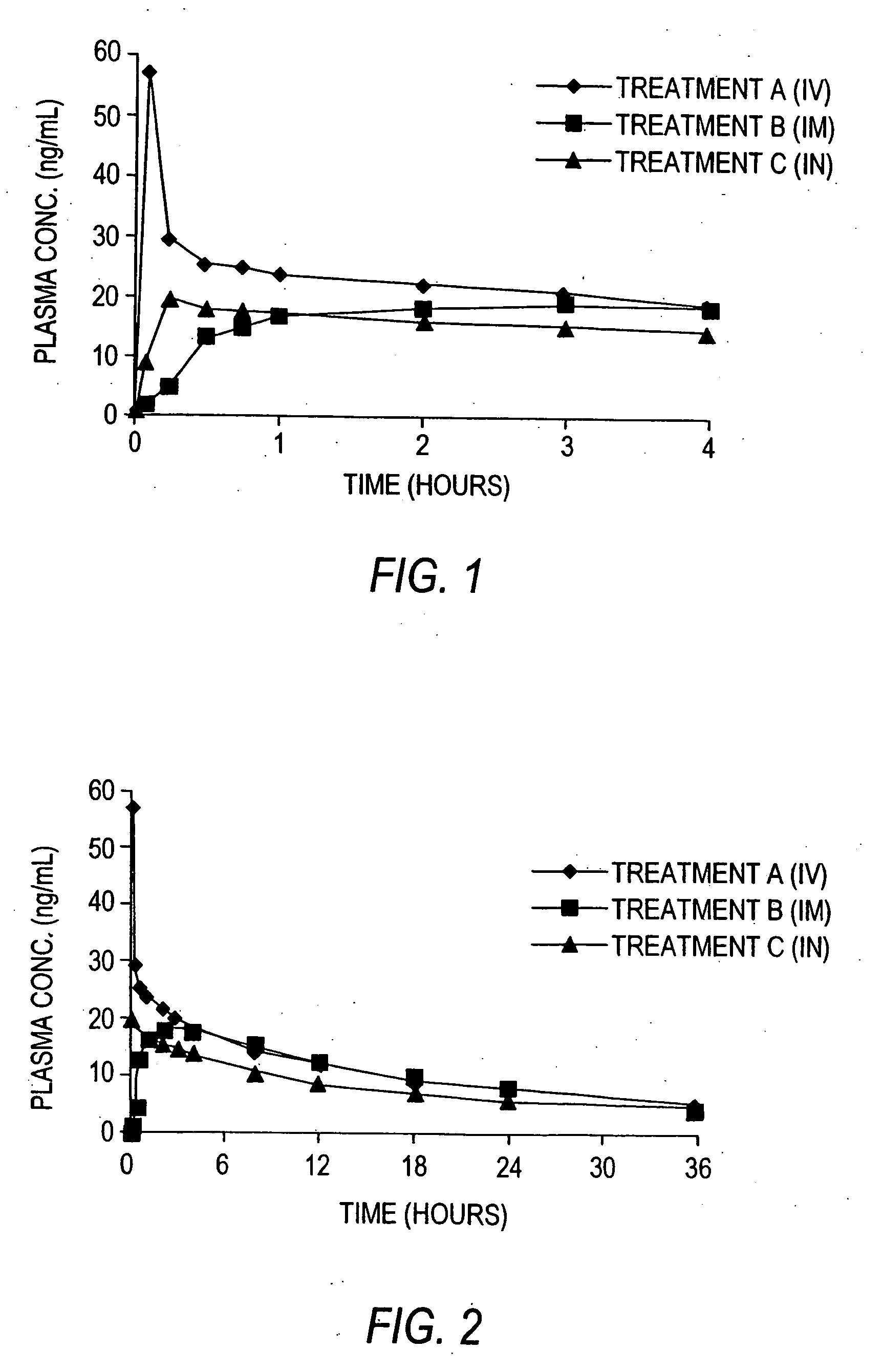
[0036] A suitable liquid composition for use in a spray device for intranasal administration includes a solvent in which the desired concentration of lorazepam can be attained to provide the desired unit-dose in a total sprayed liquid volume that can be delivered by the device and accommodated and absorbed by the subject's nasal mucosa. Lorazepam is insoluble in water. A commercially available injectable composition approved by the FDA and sold by Wyeth Laboratories under the brand name Ativan®, includes 2 mg of lorazepam in 0.18 mL of polyethylene glycol 400 in propylene glycol with 2.0% benzyl alcohol as a preservative. This composition was not acceptable for intranasal spray administration because benzyl alcohol is irritating to the mucosa.
[0037] A suitable composition for use in the invention was prepared as follows.
[0038] Lorazepam was formulated in a liquid composition for use in the practice of the invention. Since lorazepam is insoluble in water, the lorazepam was dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com