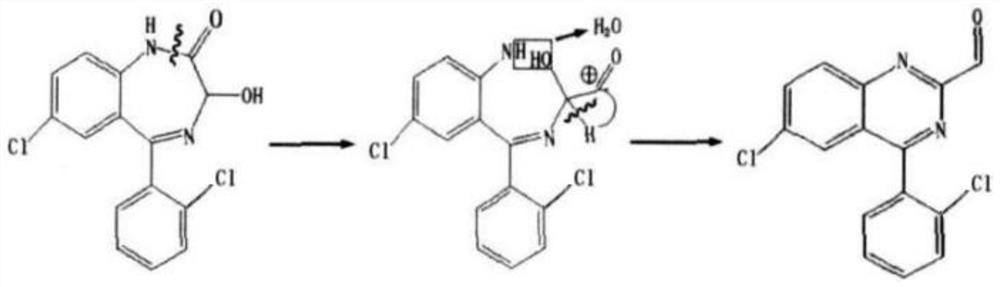
Preparation process of lorazepam impurity C
A lorazepam preparation technology, applied in the field of preparation of chemical drug lorazepam impurity C, to achieve high purity, improve preparation efficiency, and shorten the preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
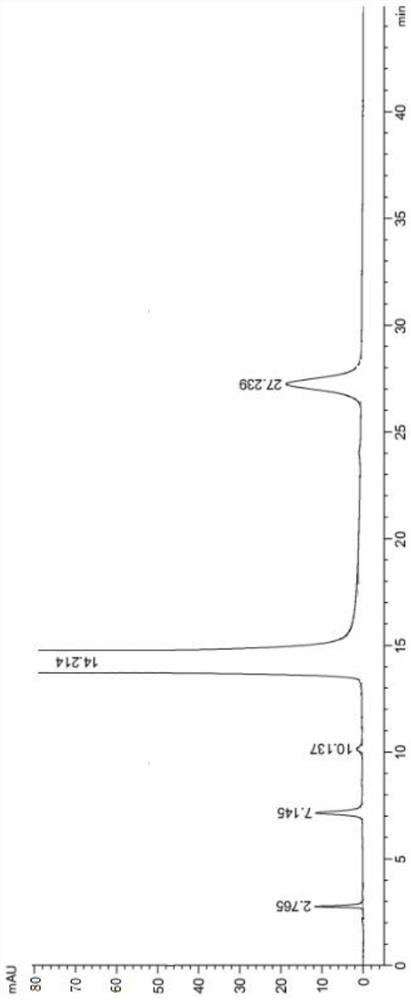
[0030] Add 500ml of toluene, 10g of lorazepam, 0.8g of p-toluenesulfonic acid, and 0.5ml of triethylamine into the reaction bottle, stir and heat up, and keep the reaction at 65°C for 8 hours. The temperature was lowered to 25° C., and the crude product solution of lorazepam impurity C was obtained by filtration, and the HPLC purity of the crude product was analyzed to be 97.13%.
[0031] The crude product of lorazepam impurity C was analyzed by HPLC. The injection conditions were as follows: Agilent ZORBAX Extend C18 column (250mm×4.6mm, 5 μm) chromatographic column was used, with 0.02mol L -1 Dipotassium hydrogen phosphate solution (1mol L -1 Sodium hydroxide adjusted the pH to 10.5)-acetonitrile (60:40) as mobile phase, flow rate 1.0mL min -1 , the column temperature was 35° C., the detection wavelength was 235 nm, and the injection volume was 10 μL.
[0032] The crude product HPLC spectrum of lorazepam impurity C is as follows figure 2 As shown, the information of each...
Embodiment 2
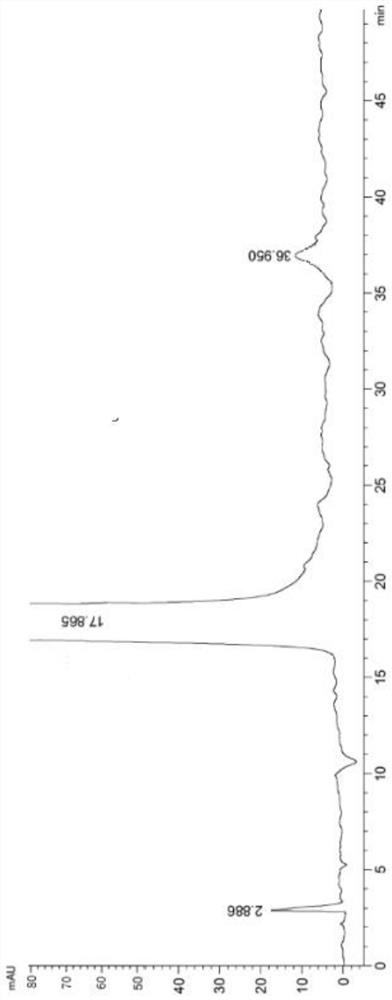
[0043] Add 200ml of ethyl acetate, 10g of lorazepam, 0.8g of p-toluenesulfonic acid, and 0.5ml of triethylamine into the reaction bottle, stir and heat up, and keep the reaction at 50°C for 11 hours. Cool down to 22°C, wash with 150ml×3 saturated brine until neutral, dry over anhydrous sodium sulfate, filter out sodium sulfate, concentrate the filtrate under reduced pressure, replace with 10ml×2 ethanol, add 15ml of isopropyl ether, freeze and filter , drying to obtain 5.5g lorazepam impurity C, HPLC purity 99.47%.
Embodiment 3
[0045] Add 200ml of ethyl acetate, 10g of lorazepam, 0.8g of p-toluenesulfonic acid, and 0.5ml of triethylamine into the reaction flask, stir and heat up, and keep the reaction at 70°C for 9 hours. Cool down to 20°C, wash with 150ml×3 saturated brine until neutral, dry over anhydrous sodium sulfate, filter out sodium sulfate, concentrate the filtrate under reduced pressure, replace with 10ml×2 methanol, add 15ml of isopropyl ether, freeze and filter , drying to obtain 5.2g lorazepam impurity C, HPLC purity 99.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com