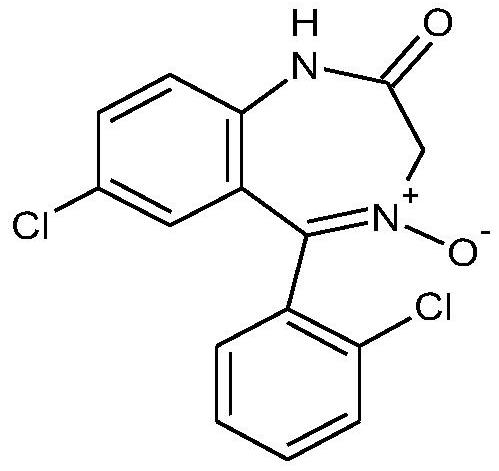
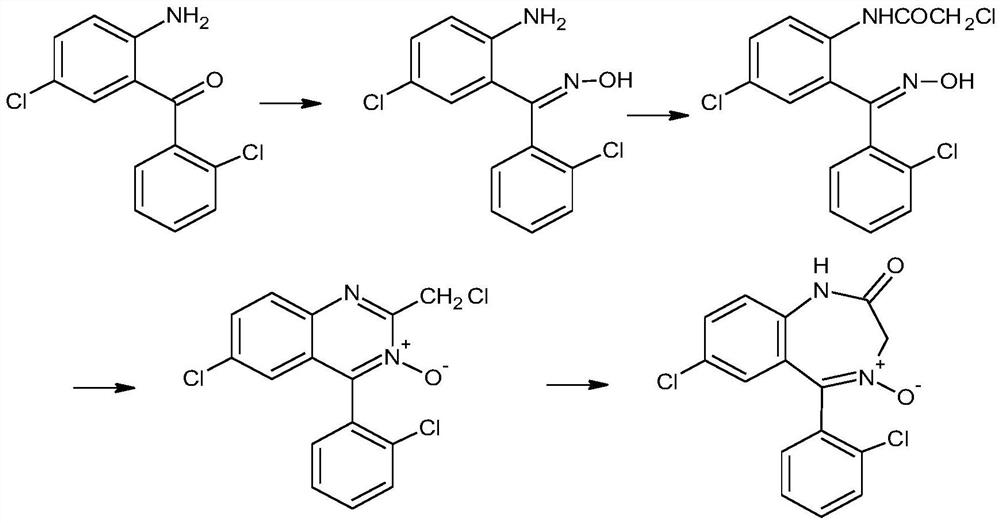
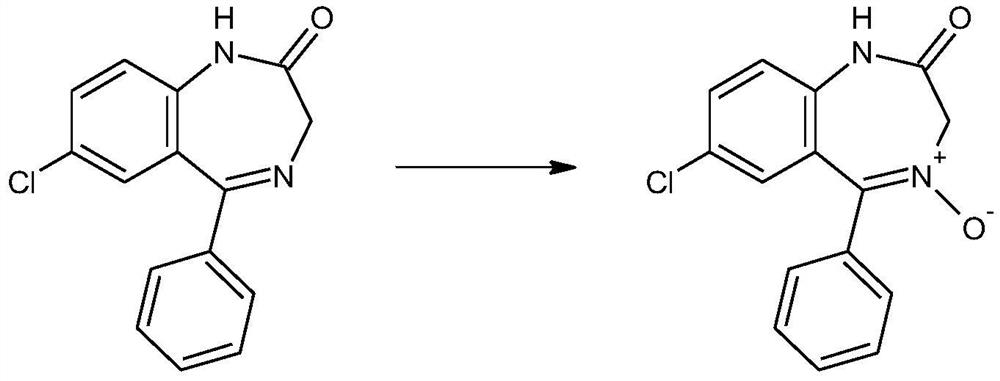
Preparation method of lorazepam intermediate
An intermediate, chlorophenyl technology, applied in the field of medicine and chemical industry, can solve the problems of limited reaction temperature, slow reaction rate, weakened oxidation, etc., to achieve the effect of reducing production cost, shortening process cycle, and improving the production site environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 30g of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepine-2-one and 75ml of dimethylformazine to the reaction flask in turn Amide, stir to dissolve and heat up to 40°C, then add 30g of hydrogen peroxide dropwise at a temperature of 40°C to 55°C with a concentration of 50wt%. After the dropwise addition was completed, the reaction was maintained at 55°C to 65°C for 3 hours. After the heat preservation reaction is completed, control the temperature at 60°C to 65°C and add 10g of hydrogen peroxide with a concentration of 50% by mass dropwise again. Cool down to -5°C, add 30g of water dropwise, continue stirring at 0°C to -5°C for 0.5 hours, let stand for 2 hours, filter, and wash with water to obtain 7-chloro-2-oxo-5-(2-chloro Phenyl)-1,4-benzodiazepine-4-oxide crude. The crude product was added into a mixed solvent of 90ml of acetone and 30ml of water for beating and refining to obtain 27.2g of 7-chloro-2-oxo-5-(2-chlorophenyl)-1,4-benzodiazepine-4- Oxid...
Embodiment 2
[0039] Add 30g of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one and 105ml of dimethylacetamide to the reaction flask in turn Amide, stir to dissolve and heat up to 40°C, then add 30g of 50% hydrogen peroxide dropwise at a controlled temperature of 40°C to 55°C. After the dropwise addition was completed, the reaction was maintained at 55°C to 65°C for 3 hours. At the end of the heat preservation reaction, control the temperature at 60°C to 65°C and add 10g of hydrogen peroxide with a concentration of 50% by mass dropwise again. After the addition is complete, continue the heat preservation reaction at 60°C to 65°C for 7 hours. Cool down to -5°C, add 50g of water dropwise, continue stirring at 0°C to -5°C for 0.5 hours, let stand for 2 hours, filter, and wash with water to obtain 7-chloro-2-oxo-5-(2-chloro Phenyl)-1,4-benzodiazepine-4-oxide crude. Add the crude product to a mixed solvent of 60ml of acetone and 15ml of water for beating and refining to obta...
Embodiment 3
[0041] Add 30g of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one and 105ml of dimethylacetamide to the reaction flask in turn Amide, stir to dissolve and heat up to 40°C, then add 30g of 50% hydrogen peroxide dropwise at a controlled temperature of 40°C to 55°C. After the dropwise addition was completed, the reaction was maintained at 55°C to 65°C for 3 hours. After the heat preservation reaction is completed, control the temperature at 60°C to 65°C and add 15g of hydrogen peroxide with a concentration of 50% by mass dropwise again. After the dropwise addition, continue the heat preservation reaction at 60°C to 65°C for 6.5 hours. Cool down to -5°C, add 54g of water dropwise, continue stirring at 0°C to -5°C for 0.5 hours, let stand for 2 hours, filter, and wash with water to obtain 7-chloro-2-oxo-5-(2-chloro Phenyl)-1,4-benzodiazepine-4-oxide crude. Add the crude product to a mixed solvent of 60ml of acetone and 15ml of water to obtain 27.7g of 7-chloro-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com