A kind of preparation method of linaclotide
A technology of linaclotide and peptidyl peptide, which is applied in the field of linaclotide preparation, can solve the problems of increased production cost, unfavorable large-scale production, cumbersome operation steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of preparation method of linaclotide, comprising the following steps:
[0029] Provide crude linaclotide line peptide;
[0030] dissolving the crude linaclotide line peptide in an ammonium chloride solution, and adjusting the pH value of the obtained system to 7.5-9.5 with an alkaline reagent to obtain a linaclotide line peptide solution;
[0031] Mixing the linaclotide linear peptide solution with an aqueous hydrogen peroxide solution, continuously feeding air into the resulting reaction solution for oxidation reaction, to obtain an oxidation product system;
[0032] The oxidation product system is freeze-dried to obtain linaclotide.
Embodiment 1
[0043] Preparation of crude linaclotide line peptide, comprising the following steps:
[0044] Take Fmoc-Tyr(tBu)-Wang Resin 140.12g (100mmol) with a degree of substitution of 0.70mmol / g; wherein, Fmoc is 9-fluorenylmethoxycarbonyl, Wang Resin is 4-benzyloxybenzyl alcohol resin, and tBu is tertiary Butyl), added in the solid-phase reaction column, after swelling the resin with N,N-dimethylformamide (DMF) for 60min, vacuum filtration, adding 3 times the mixture of piperidine and DMF of the resin bed volume (the The volume fraction of piperidine in the mixture is 20%), stirred for 30min to remove Fmoc protection, and washed 5 times with DMF after vacuum filtration;
[0045] Dissolve 176.3g (300mmol) Fmoc-Cys(Trt)-OH and 40.5g (300mmol) 1-hydroxybenzotriazole (HOBt) in DMF and cool at -10°C for 20min, add 47.3mL (300mmol) N ,N-diisopropylcarbodiimide (DIC) and continue to activate for 30min; put the activated reaction solution into the solid-phase reaction column, and carry out ...
Embodiment 2
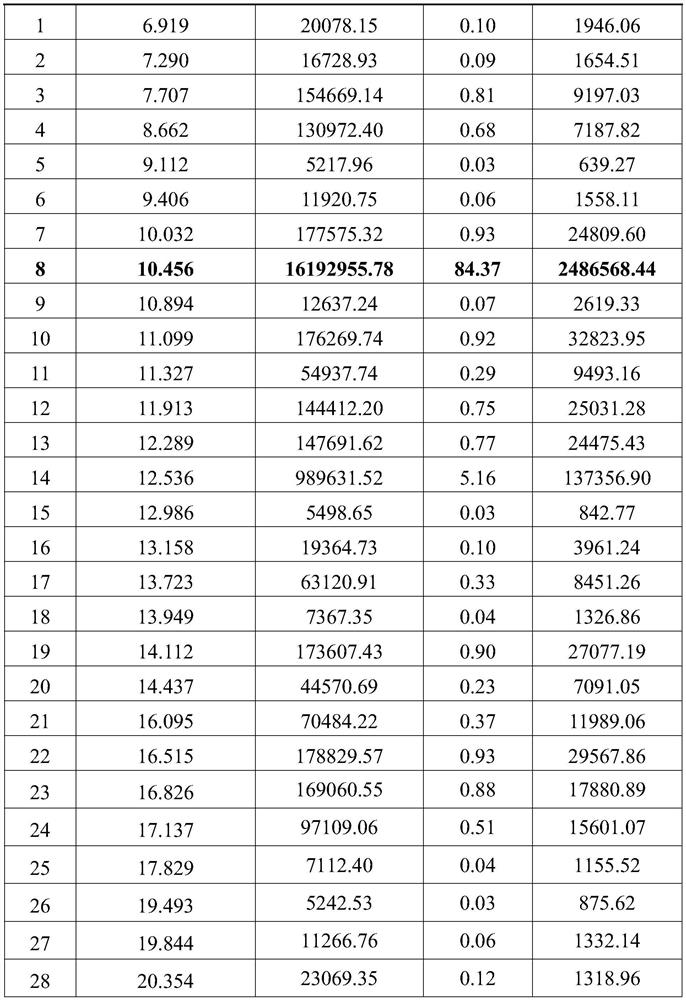
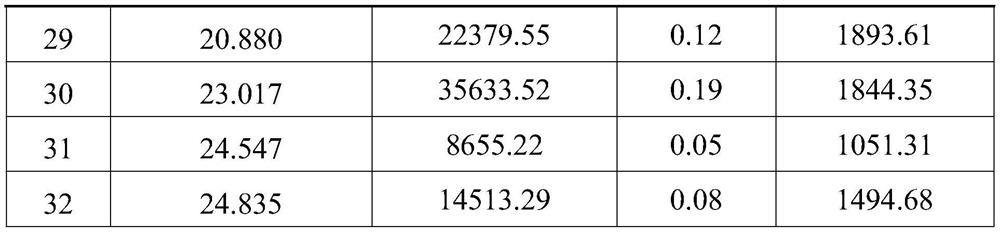
[0057] Weigh 1g of linaclotide line peptide crude product (purity is 84.37%), ultrasonically dissolve in 4L ammonium chloride solution with a concentration of 0.05mol / L, adjust the pH value to 8.0 with ammonia water with a concentration of 0.5wt%, and then add 900 μL of hydrogen peroxide with a concentration of 10wt% was continuously fed into the reaction solution obtained by magnetic stirring at room temperature (25° C.) to carry out the oxidation reaction for 4 hours, wherein the air was specifically fed into the reaction through a corrosion-resistant hose In the liquid, the air pressure passed into the flexible pipe is 0.01MPa (after deducting the atmospheric pressure), and the diameter of the flexible pipe is 8mm; after the oxidation reaction finishes, the purity of the resulting product system is 72.92% (HPLC figure is shown image 3 As shown, the unit of the abscissa is min, and the unit of the ordinate is AU; the data information corresponding to the chromatographic peak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com