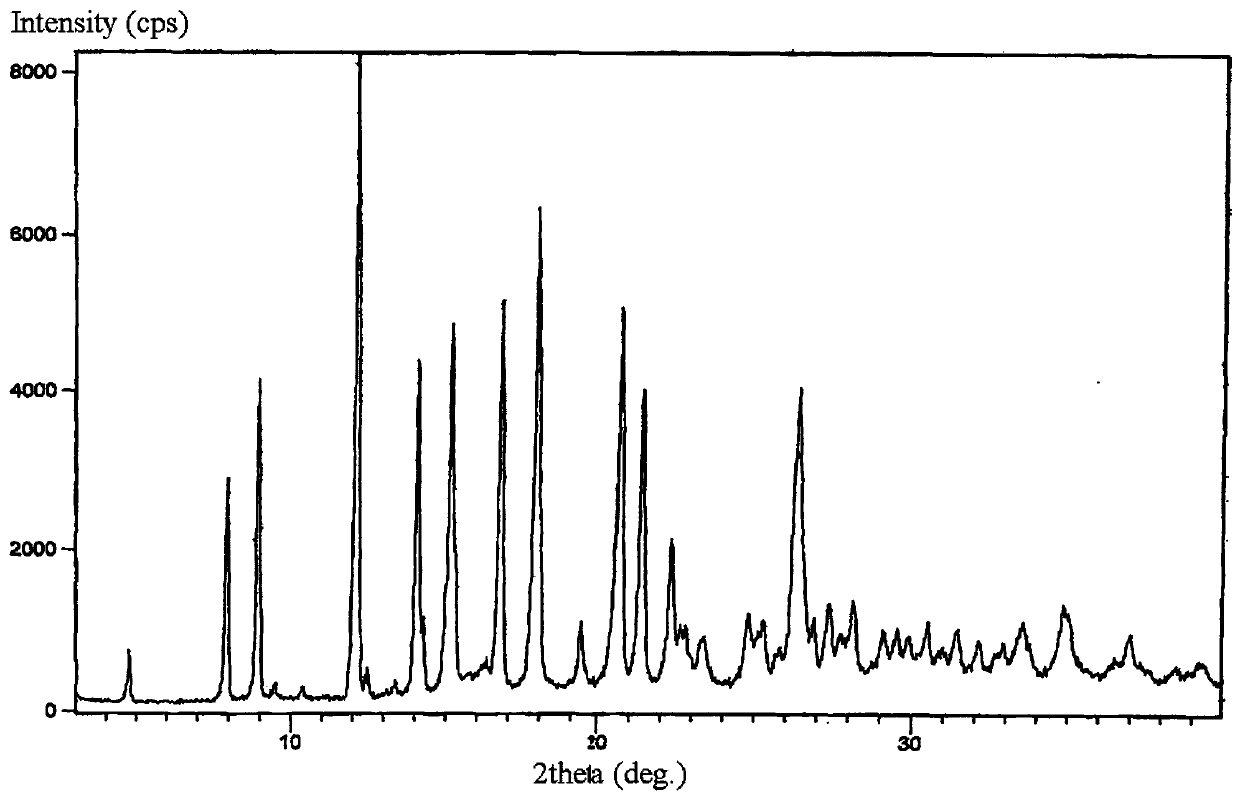
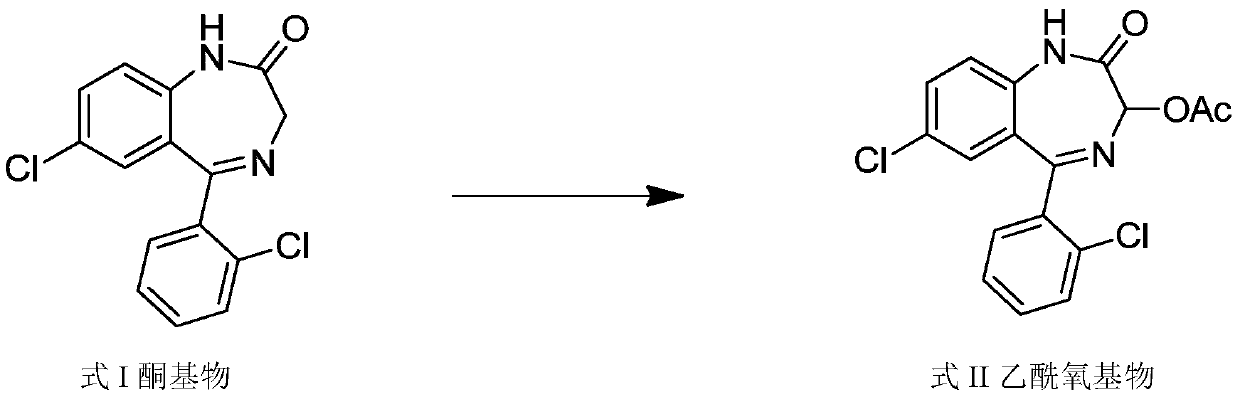
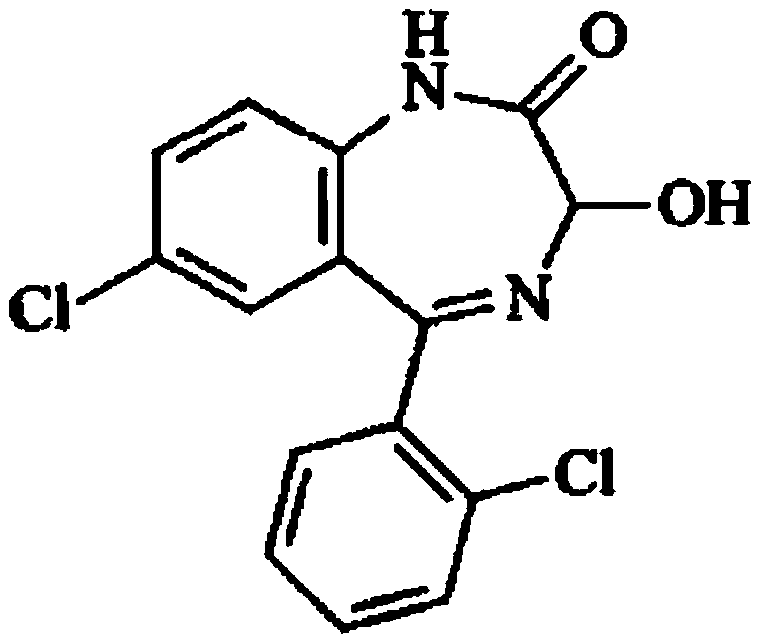
Method for preparing lorazepam
The technology of lorazepam and crystallization is applied in the field of preparation of benzodiazepine drugs lorazepam, and can solve the problems of affecting the long-term storage and transportation of preparations, being unstable when exposed to light, and being low in oral bioavailability of the drug.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: Preparation of lorazepam
[0070] Step 1, acetoxylation reaction prepares the acetoxy compound of formula II
[0071] Add 1 mol (305 g) of the ketone base of formula I, 14 mol of glacial acetic acid, 2.5 mol of potassium acetate, 1.8 mol of potassium persulfate, and 2.5 mol of iodine into the reaction flask, stir at 80°C to react for 7 hours, then distill under reduced pressure at 65°C Remove the acid solution; then add ethyl acetate (4 times the amount of the ketone base), 5% sodium thiosulfate solution (6 times the amount of the ketone base) in the reaction flask, stir for 25 minutes, let stand layer; collect the organic layer, extract the aqueous layer twice with ethyl acetate, combine the organic layers, add 1.25 times the volume of saturated sodium chloride solution therein, stir for 25 minutes, let stand to separate the layers, and separate the organic layer; the organic layer Add medicinal charcoal (0.75%), stir and decolorize at 65°C for 25 minu...
Embodiment 2
[0076] Embodiment 2: Preparation of lorazepam
[0077]Step 1, acetoxylation reaction prepares the acetoxy compound of formula II
[0078] Add 1 mol (305 g) of ketones of formula I, 15 mol of glacial acetic acid, 2 mol of potassium acetate, 2 mol of potassium persulfate, and 2 mol of iodine into the reaction flask, stir at 70°C to react for 8 hours, then distill off the acid solution at 70°C under reduced pressure Then add ethyl acetate (3 times of the ketone-based charging capacity), 5% sodium thiosulfate solution (7 times of the ketone-based charging capacity) in the reaction flask, stir for 20 minutes, leave standstill and make layering; collect Extract the organic layer and the aqueous layer twice with ethyl acetate, combine the organic layers, add 1 times the volume of saturated sodium chloride solution to it, stir for 20 minutes, let stand to separate the layers, and separate the organic layer; the organic layer is used for medicinal purposes Charcoal (0.5%), stirred a...
Embodiment 3
[0083] Embodiment 3: Preparation of lorazepam
[0084] Step 1, acetoxylation reaction prepares the acetoxy compound of formula II
[0085] Add 1 mol (305 g) of ketones of formula I to the reaction flask, 12 mol of glacial acetic acid, 3 mol of potassium acetate, 1.5 mol of potassium persulfate, and 3 mol of iodine, stir at 90°C to react for 6 hours, then distill off the acid at 60°C under reduced pressure solution; then add ethyl acetate (5 times of the ketone-based feeding amount), 5% sodium thiosulfate solution (5 times of the ketone-based feeding amount) in the reaction flask, stir for 30 minutes, and let stand to make stratification; Collect the organic layer, extract the aqueous layer twice with ethyl acetate, combine the organic layers, add 1.5 times the volume of saturated sodium chloride solution to it, stir for 30 minutes, let stand to separate the layers, and separate the organic layer; add medicine to the organic layer Use charcoal (1%), stir and decolorize at 60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com