Preparation method of lorazepam intermediate
An intermediate, chlorophenyl technology, applied in the field of preparation of lorazepam intermediates, can solve the problems of low total reaction yield, cumbersome post-processing operations and the like, achieves high yield, easy separation and purification, and low environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
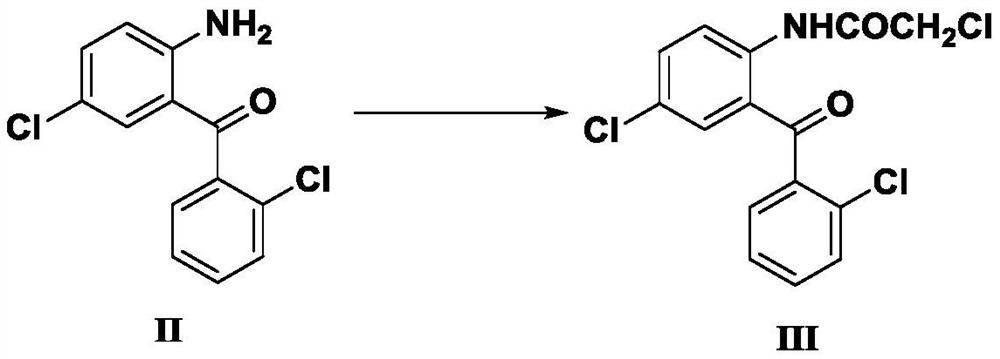
[0068] Example 1 : Preparation of 2-chloroacetamido-2', 5-dichlorobenzophenone (III)
[0069] Add 150g of 2-amino-2',5-dichlorobenzophenone (II), 124.6g of anhydrous potassium carbonate and 1200ml of ethyl acetate into a 3L dry three-necked flask, and cool down to 10°C in an ice-water bath About 82.8g of chloroacetyl chloride was added dropwise, and the temperature was controlled at 10-20°C during the dropwise addition. After the dropwise addition, the reaction was stirred at a temperature of 10±5°C, and the reaction progress was monitored by thin-layer chromatography (TLC). After stirring the reaction for 1 hour, the reaction appeared to be complete.
[0070] After the reaction was completed, 1000ml of water was added to the reaction bottle, stirred for 30 minutes, and suction filtered. The filtered product was air-dried at 80° C. for 4 hours to obtain 185.5 g of light yellow solid powder.
[0071] The light yellow solid powder was analyzed by proton nuclear magnetic reson...
Embodiment 2
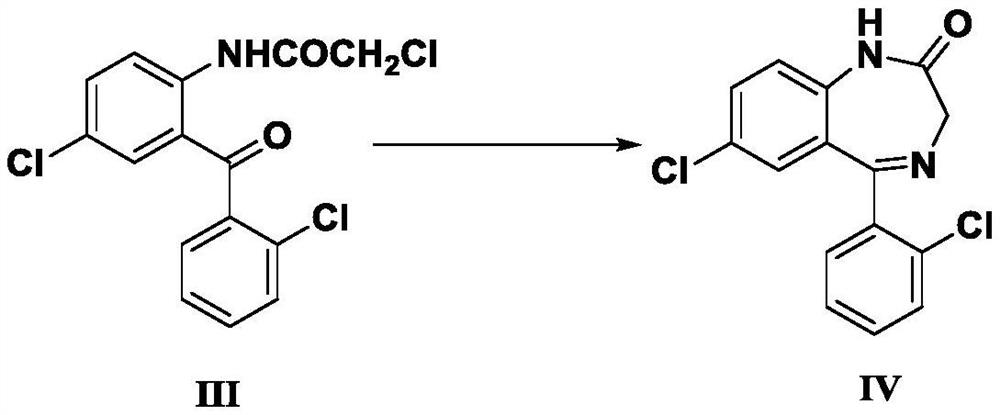
[0072] Example 2 : Preparation of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one (IV)
[0073] Add 2-chloroacetamido-2',5-dichlorobenzophenone (III), 162g of urotropine, 89g of ammonium acetate and 1800ml of 95% ethanol, heated to reflux. After about 30 minutes at reflux, the solid dissolved to give a clear yellow solution. The reflux reaction was continued, and the progress of the reaction was monitored by thin-layer chromatography (TLC). After 3 hours of reflux reaction, it was shown that the reaction was complete.
[0074] After the reaction was completed, about 1350ml of ethanol was removed by atmospheric distillation. Add 500ml of water to the distilled product, stir at room temperature for 30 minutes, and filter with suction. The filtered product was air-dried at 80° C. for 4 hours to obtain 136.28 g of off-white solid powder.
[0075] The white solid powder was analyzed by proton nuclear magnetic resonance, and the result showed that the white ...
Embodiment 3
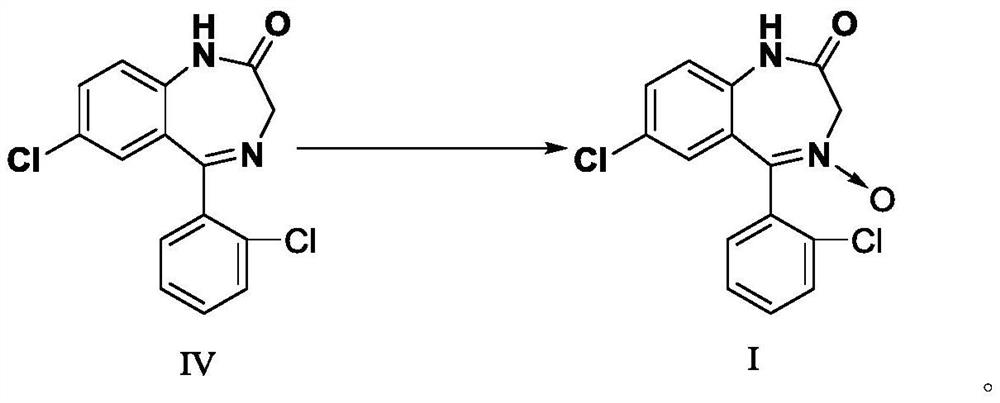
[0076] Example 3 : Preparation of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one-4-oxide (I)
[0077] Add 130g of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one prepared in Example 2 into a 2L three-necked flask (IV), 1040ml of glacial acetic acid and 193.2g of 30% by weight hydrogen peroxide were stirred and heated to 65° C., and the solid gradually dissolved. Continue heating to 80° C., keep warm and stir to react, and monitor the progress of the reaction by thin layer chromatography (TLC). After 4 hours of reaction, it shows that the reaction is complete.
[0078] After the reaction is completed, cool and stir, and add 30% by weight of sodium bisulfite solution to decompose excess hydrogen peroxide. At this time, the grayish white turbid liquid in the reaction bottle. Add 260ml of water, stir for 1 hour, and filter with suction. The filtered product was air-dried at 80° C. for 4 hours to obtain 118.64 g of beige solid powder.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com