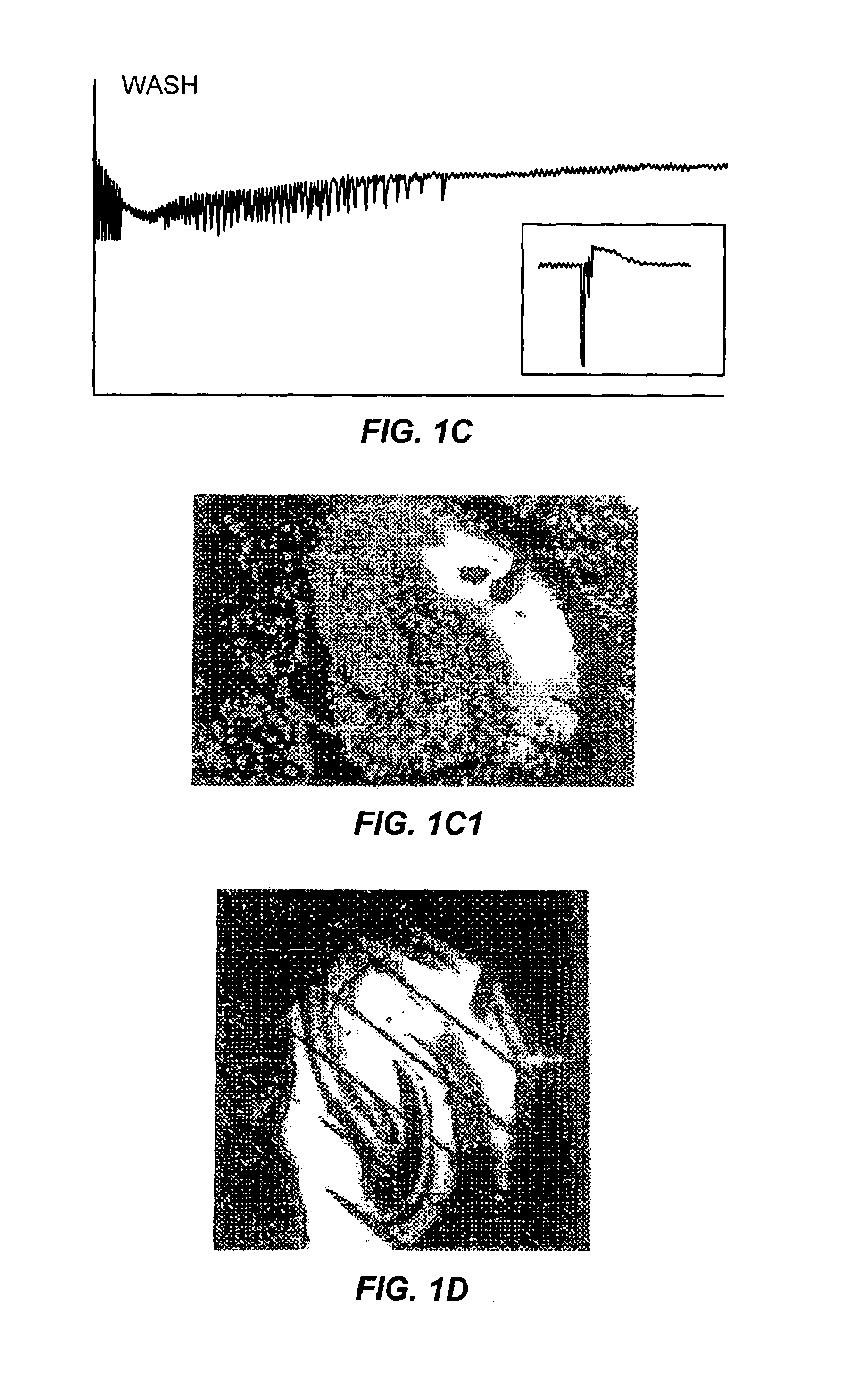
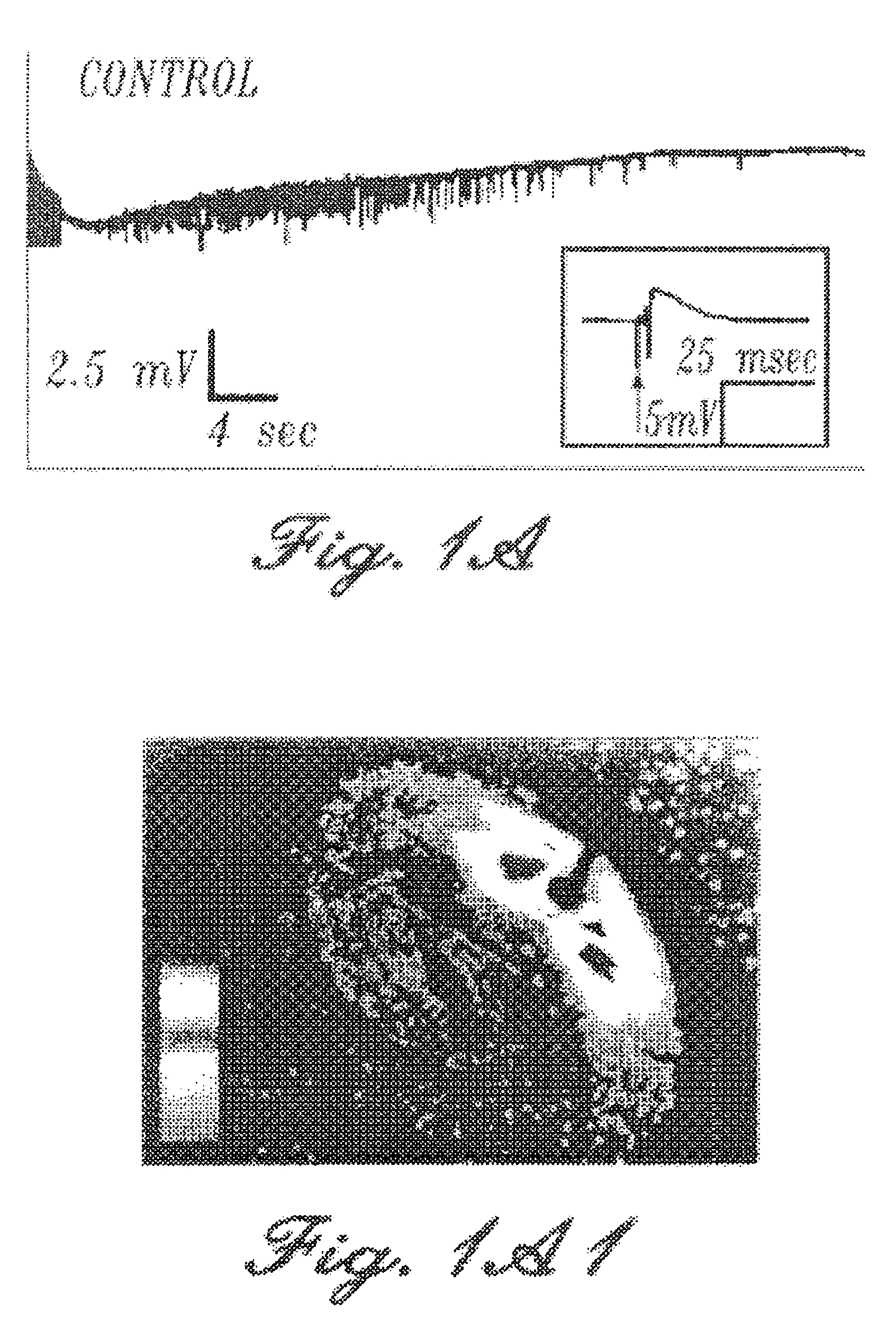
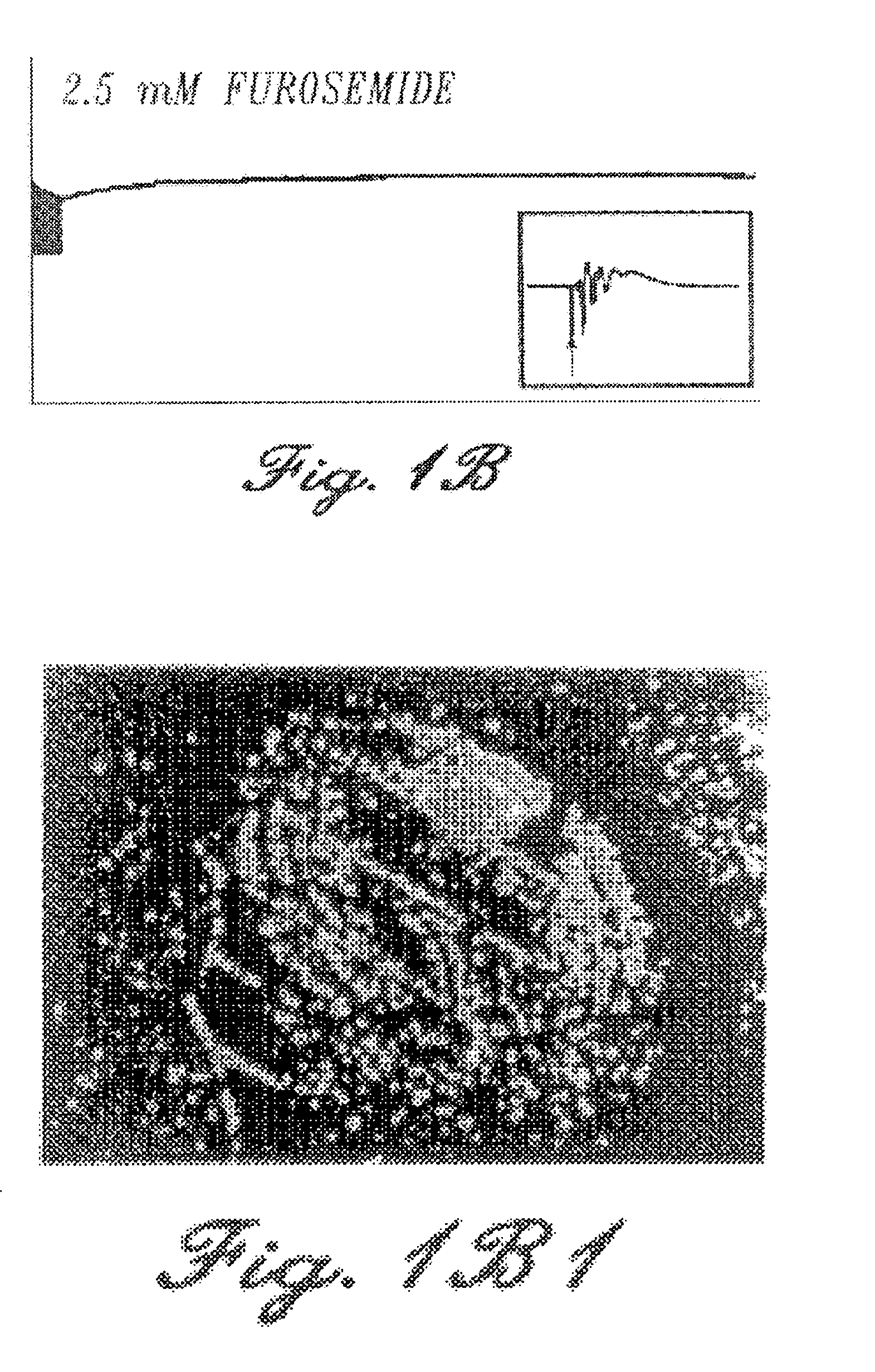
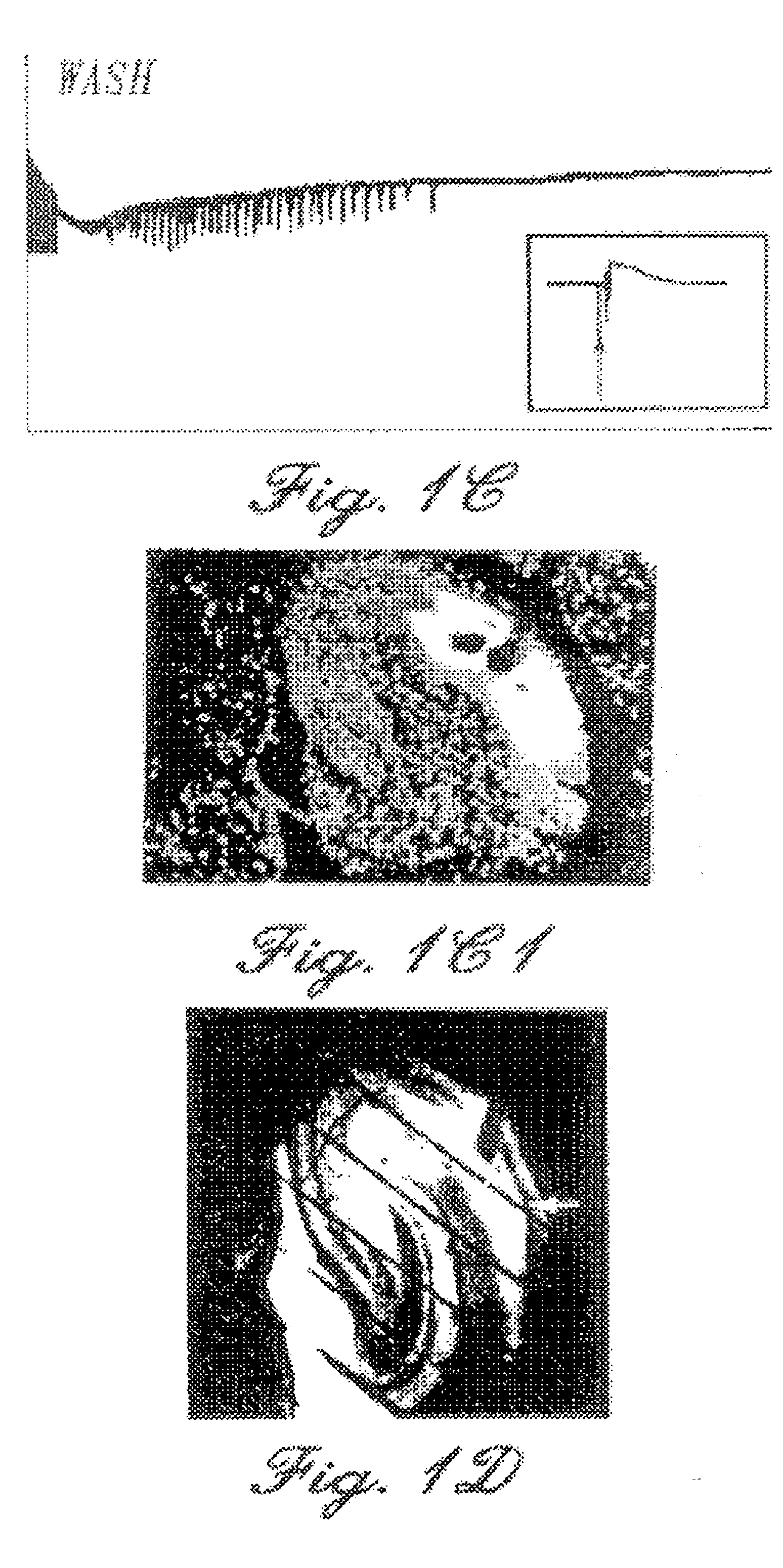
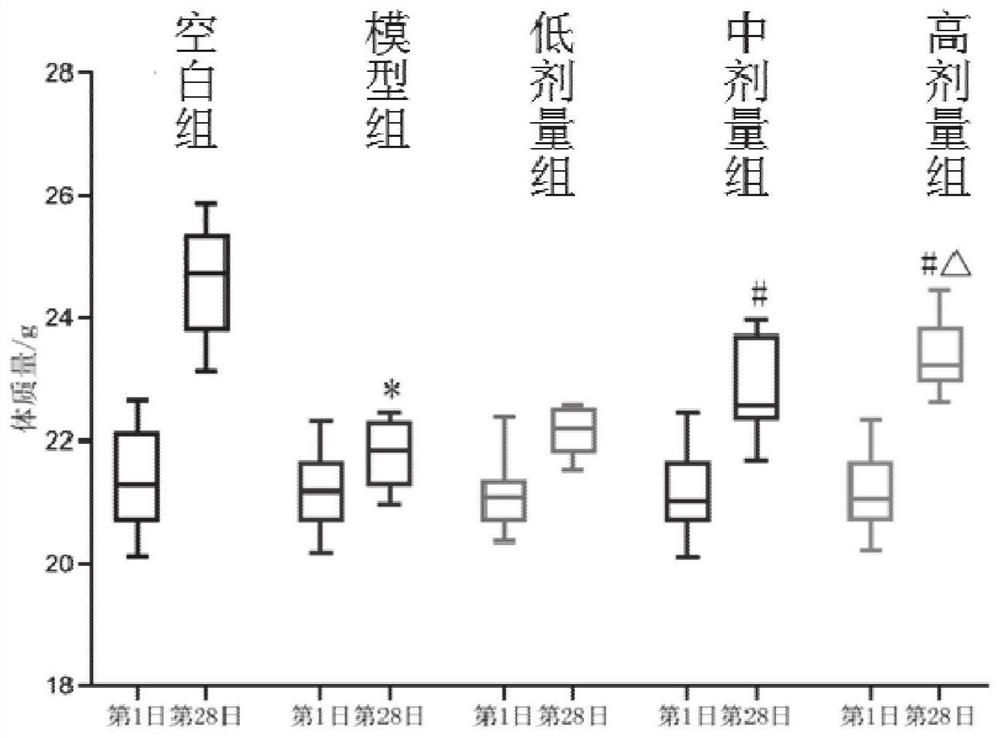
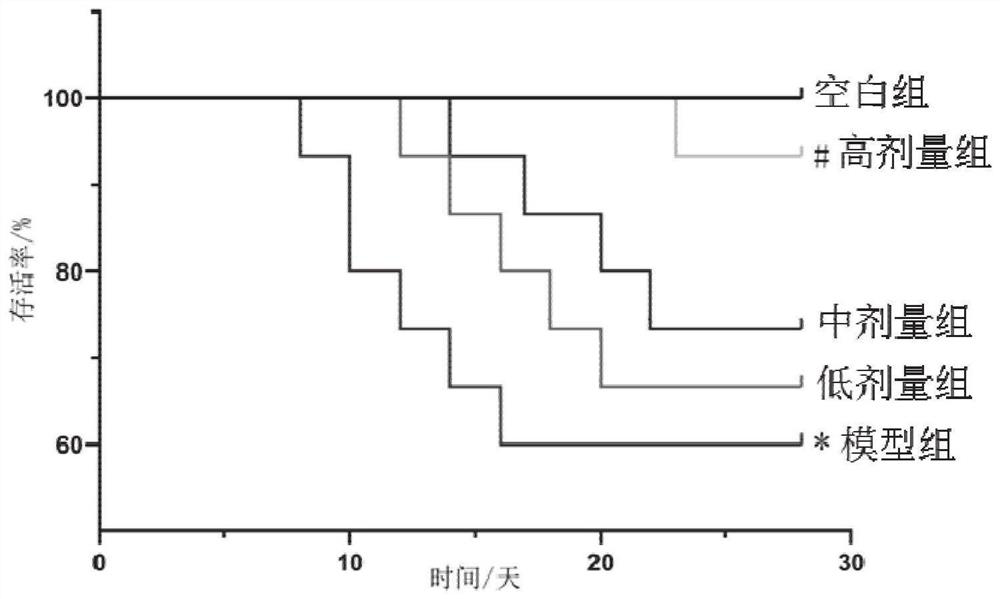
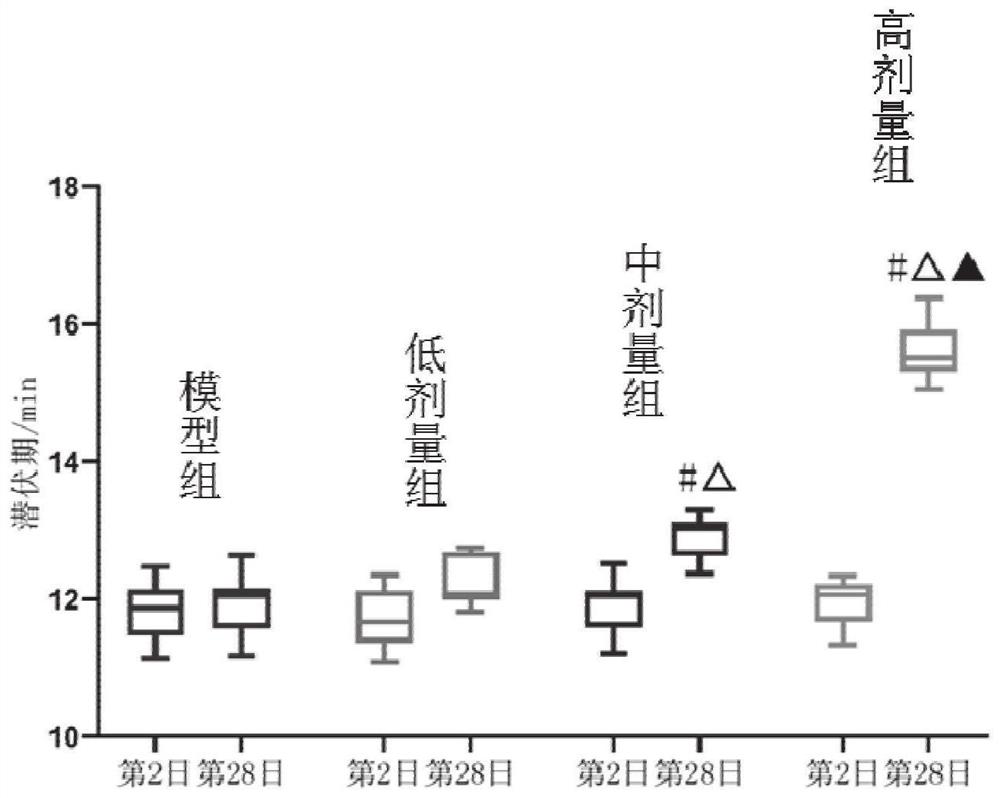
Patents
Literature
42 results about "Epilepticus status" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Status epilepticus. Status epilepticus (SE) is a single epileptic seizure lasting more than five minutes or two or more seizures within a five-minute period without the person returning to normal between them. Previous definitions used a 30-minute time limit.
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20060198896A1Easy doseReduce injection volumeBiocidePowder deliveryBenzodiazepinePolyethylene glycol
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
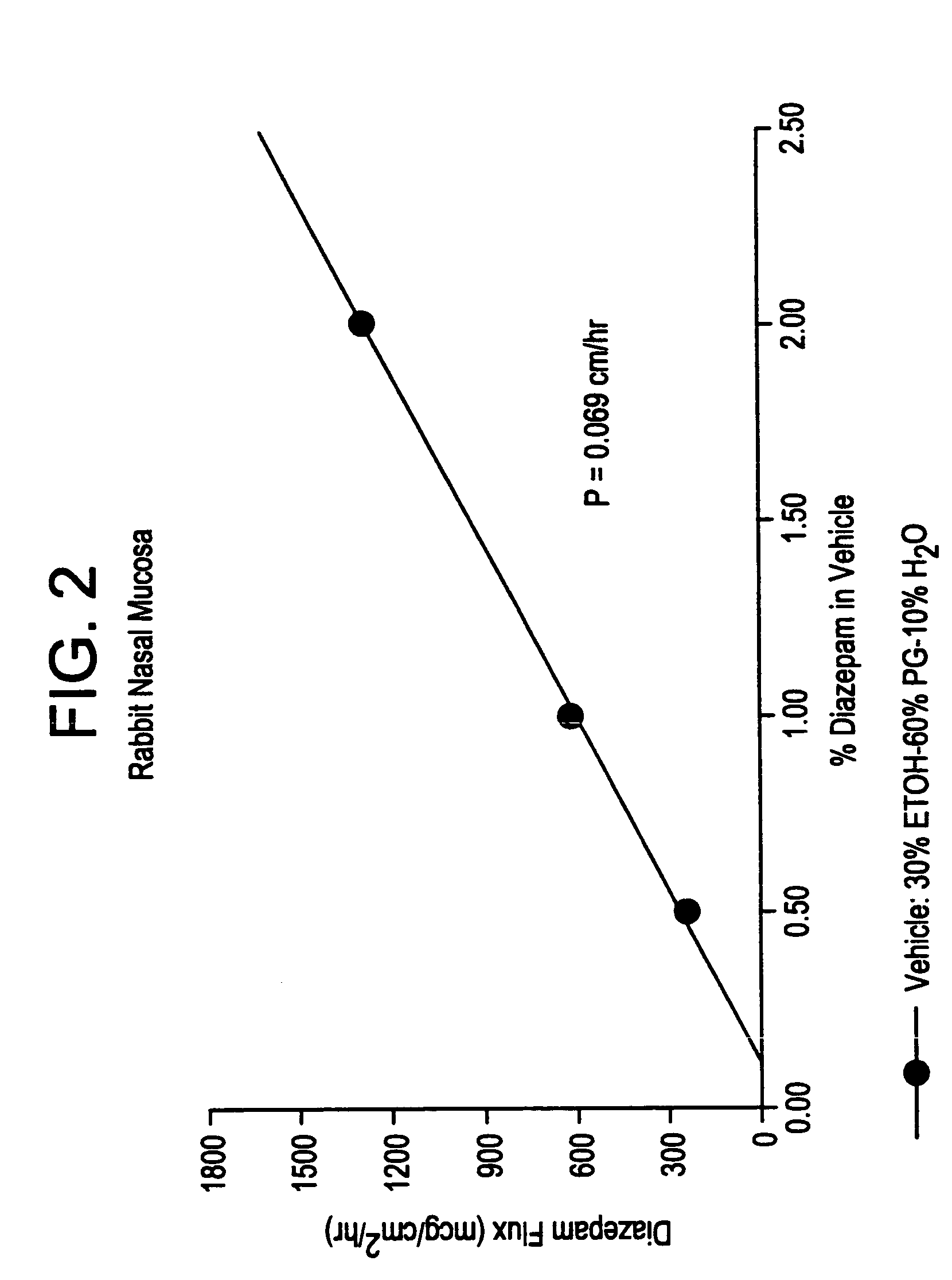
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
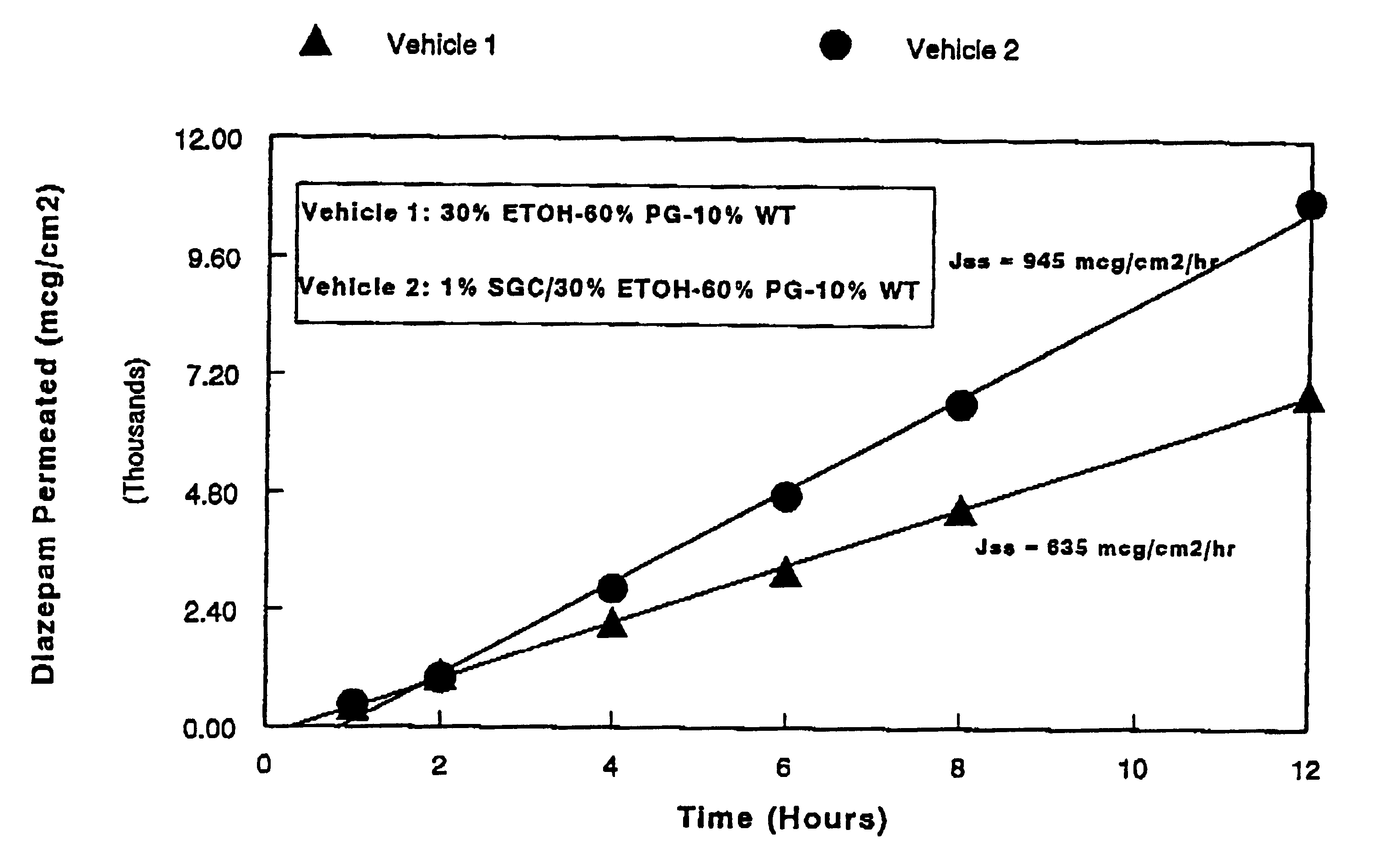
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
19-NOR neuroactive steroids and methods of use thereof
ActiveUS9365611B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderWithdrawal syndromeSubstance abuser
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I): and pharmaceutical compositions thereof. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, tinnitus, and status epilepticus.
Owner:SAGE THERAPEUTICS
Methods for treating status epilepticus and related conditions
InactiveUS20090018197A1Reduce in quantityShorten the construction periodBiocideNervous disorderSeizure clustersAnesthesia
The present invention is directed to the novel use of a class of peptide compounds for treating status epilepticus or related conditions, e.g. acute repetitive seizures, seizure clusters, etc.
Owner:UCB SA
Method of treating migraine headache without aura
InactiveUS7214711B2Function increaseUseful in treatmentBiocideNervous disorderNervous systemTreatment pain
The present invention relates to methods and compositions for treating selected conditions of the central and peripheral nervous systems employing non-synaptic mechanisms. More specifically, one aspect of the present invention relates to methods and materials for treating seizure and seizure disorders, epilepsy, status epilepticus, migraine, spreading depression, intracranial hypertension; for treating the pathophysiological effects of head trauma, stroke, ischemia and hypoxia; for treating or protecting from the pathophysiological effects of neurotoxic agents such as ethanol; and for treating neurophsyciatric disorders and central nervous system edema by administering agents that modulate ionic concentrations and / or ionic gradients in the brain, particularly ion-dependent or cation-chloride cotransporter antagonists. Electrolyte cotransport antagonists and combinations of such compositions with other agents for treating various conditions are disclosed. The present invention also relates to methods and compositions for treating pain by administering ion-dependent cotransporter antagonists. Methods and compositions for enhancing cortical function, for example, in centers of cognition, learning and memory, by administering ion-dependent cotransporter agonists are disclosed.
Owner:NEUROTHERAPEUTICS PHARMA
Compositions and methods for the treatment of disorders of the central and peripheral nervous systems
InactiveUS20060035914A1Reducing neurodegenerative effectReduced activitySalicyclic acid active ingredientsBiocideDiseaseNervous system
The present invention relates to methods and compositions for treating selected conditions of the central and peripheral nervous systems employing non-synaptic mechanisms. More specifically, one aspect of the present invention relates to methods and materials for treating seizure and seizure disorders, epilepsy, status epilepticus, migraine, spreading depression, intracranial hypertension; for treating the pathophysiological effects of head trauma, stroke, ischemia and hypoxia; for treating or protecting from the pathophysiological effects of neurotoxic agents such as ethanol; and for treating neuropsychiatric disorders and central nervous system edema by administering agents that modulate ionic concentrations and / or ionic gradients in the brain, particularly ion-dependent or cation-chloride cotransporter antagonists. Electrolyte cotransport antagonists and combinations of such compositions with other agents for treating various conditions are disclosed.
Owner:NEUROTHERAPEUTICS PHARMA
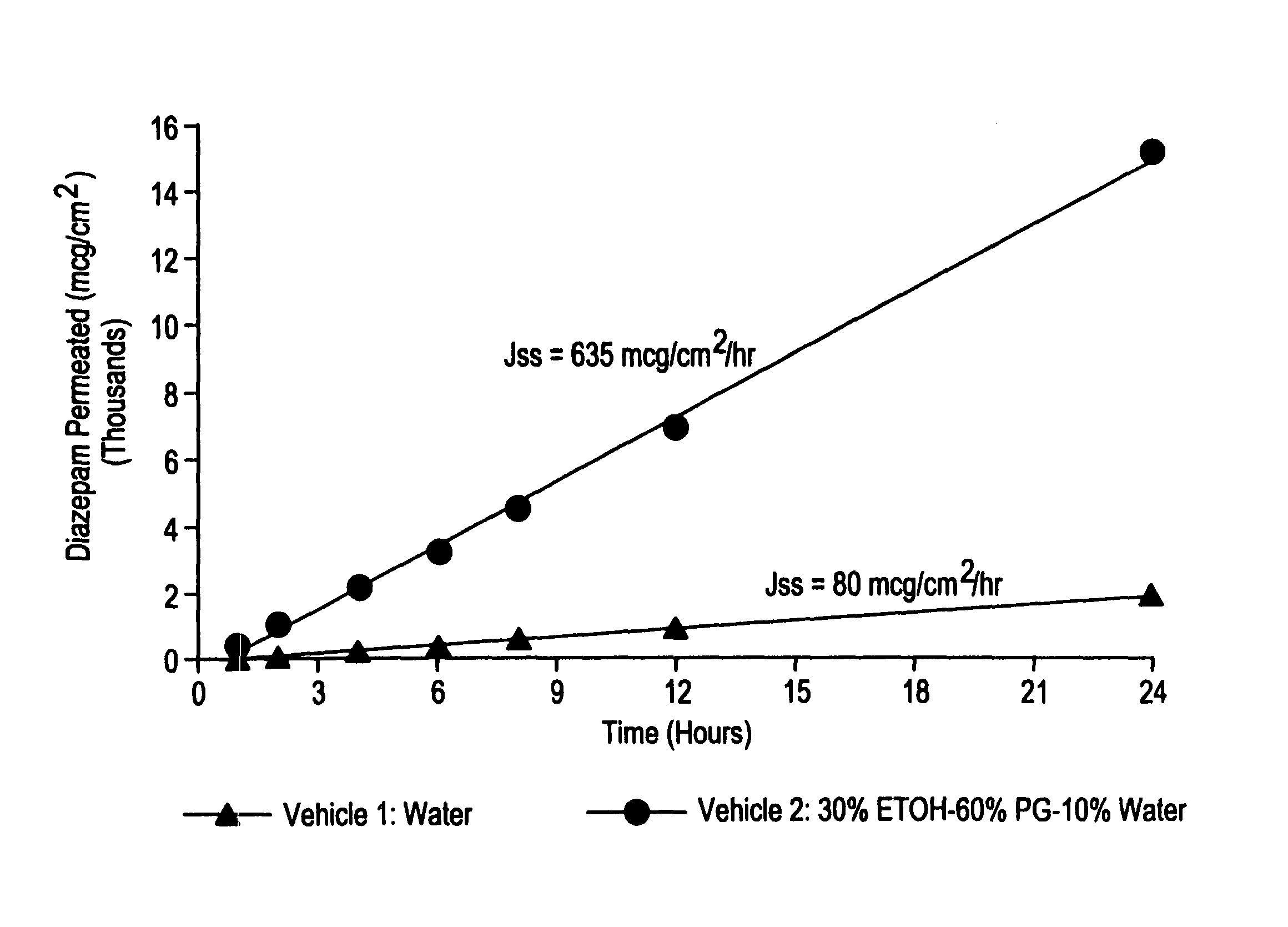
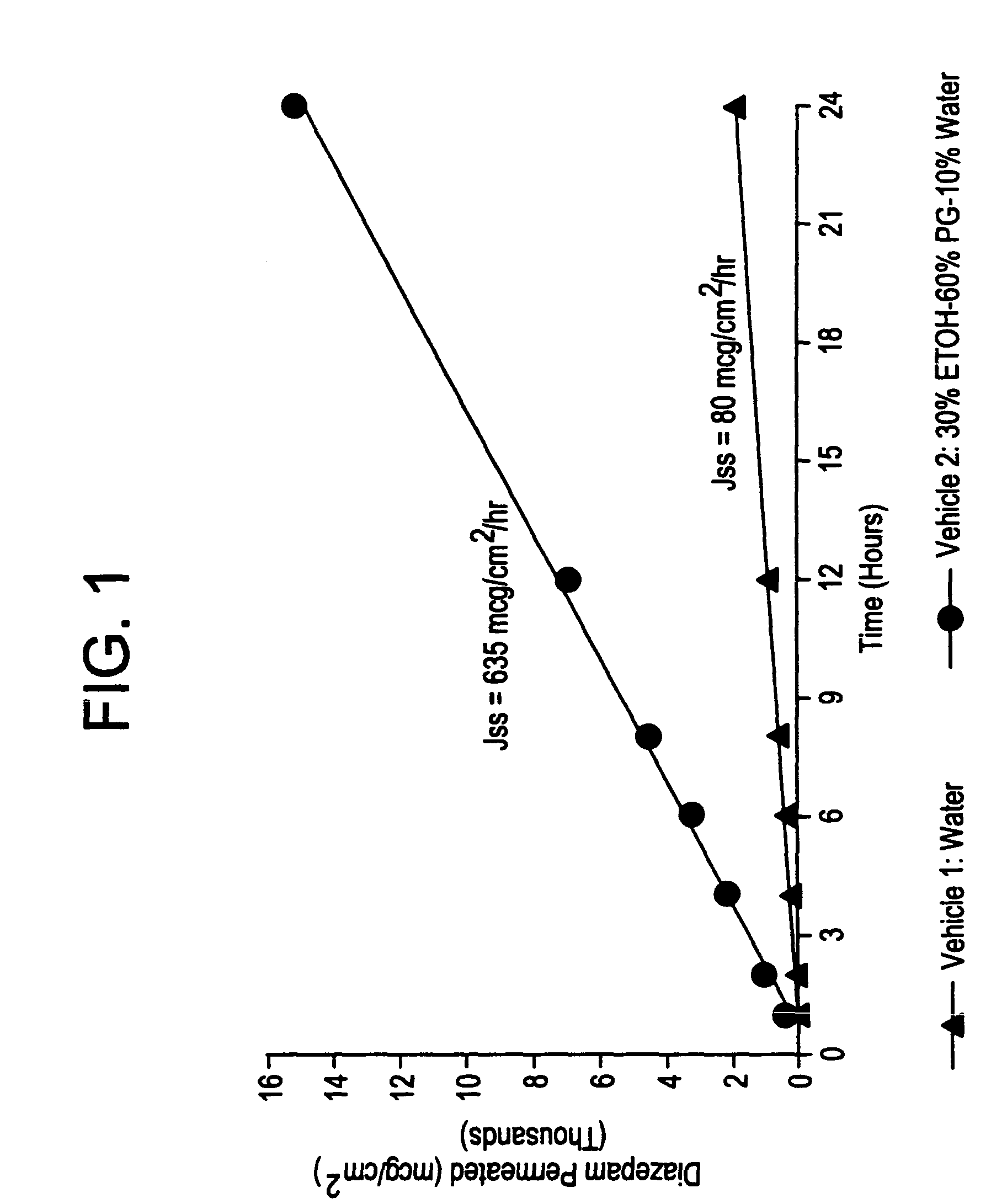
Transnasal microemulsions containing diazepam
InactiveUS20050002987A1High plasma concentrationHigh concentrationPowder deliveryNervous disorderNasal cavityBlood plasma
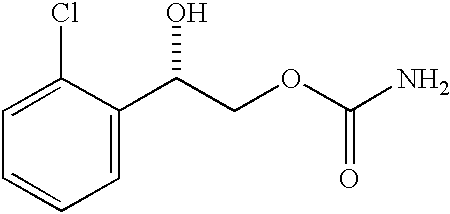
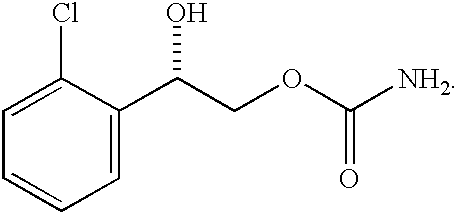
Diazepam is administered intranasally in the form of specific microemulsions having advantageous properties. The microemulsions are comprised of about equal quantities of a fatty acid and water with the remainder being a hydrophilic surfactant, a polar solvent and an alcohol in a weight ratio such that alcohol is present in a greater quantity by weight than either of the other two. Nasal administration of the subject microemulsions produces a high plasma concentration of diazepam nearly as fast as intravenous administration. The present microemulsions are particularly suitable for a prompt and timely treatment of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:SK
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20090304801A1Easy to atomizeFacilitate depositionPowder deliveryBiocideBenzodiazepineNanoparticle
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
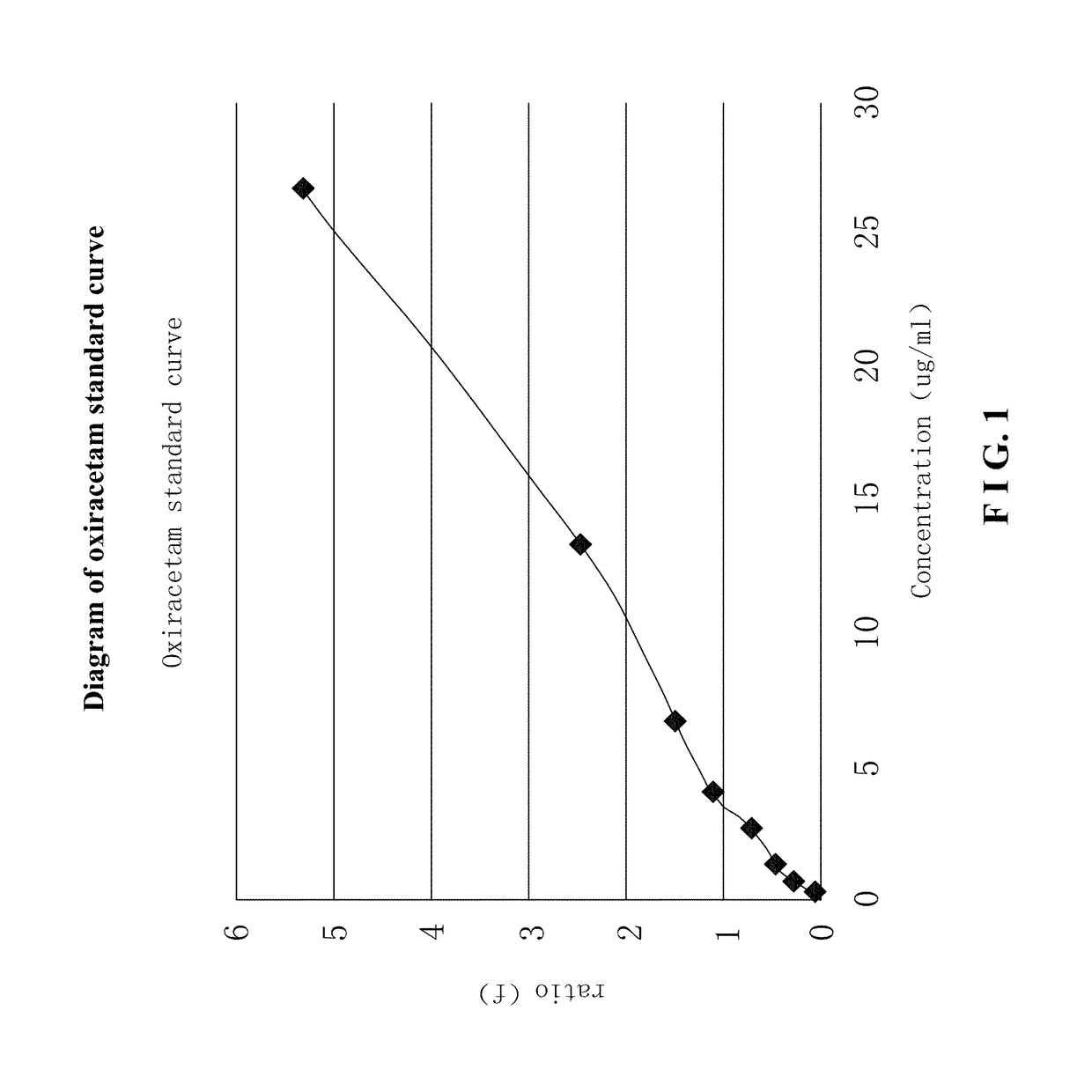
Application of dextro-oxiracetam in pharmaceutical field
InactiveCN106166150AHas antiepileptic activityImprove bioavailabilityOrganic active ingredientsNervous disorderPartial epilepsyTherapeutic effect
The invention provides application of dextro-oxiracetam in preparation of drugs for prevention or treatment of epilepsy. Experimental results show that dextro-oxiracetam has obvious treatment effect on generalized epilepsy seizure, partial epilepsy seizure and status epilepticus.
Owner:CHONGQING RUNZE PHARM CO LTD
Transnasal anticonvulsive compositions and modulated process
InactiveUS7132112B2Promote absorptionIncrease permeationNervous disorderAerosol deliveryNasal cavityCo administration
A novel method of vehicle modulated administration of an anticonvulsive agent to the mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol (10–80%) or a glycol (10–80%), and their combinations with a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Compositions and methods for treating seizures
Owner:DRAGTEK CORP
Methods and Compositions for Treating Status Epilepticus and Seizures Causing Status Epilepticus
InactiveUS20110230473A1Improve survivabilityPreventing and inhibiting and reducing seizureBiocideNervous disorderNR1 NMDA receptorNMDA receptor
Disclosed herein are methods, kits and compositions for treating, preventing, inhibiting, or reducing a seizure, status epilepticus, neuropathogenesis or a neuropathology caused by overstimulation of the NMDA receptor pathway and / or exposure to an OP compound.
Owner:GORDON RICHARD K +5
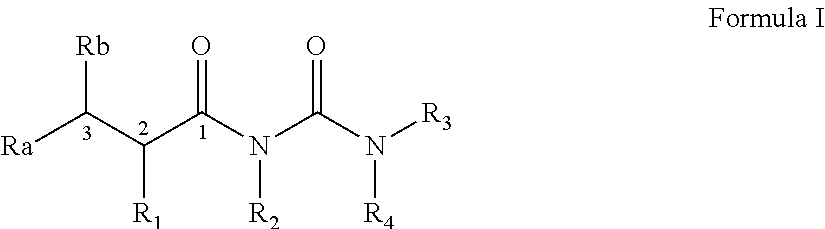
N-substituted imidazole formate derivative and application thereof
The invention discloses a compound as shown in the formula I, or a stereisomer, or pharmaceutically acceptable salt, or a solvate, or a prodrug, or a metabolite, or a deuterated derivative thereof. The compound is an N-substituted imidazole formate derivative with a novel structure and belongs to the field of medicinal chemistry. The invention also discloses application of the N-substituted imidazole formate derivative in the preparation of medicines with sedative, hypnotic and / or anesthetic effects, and application in the preparation of medicines capable of controlling the status epilepticus.The compound has a good inhibitory effect on the central nervous system, and provides a new selection for clinical screening and / or preparation of medicines with the sedative, hypnotic and / or anesthetic effects and capable of controlling the status epilepticus.
Owner:CHENGDU MFS PHARMA CO LTD
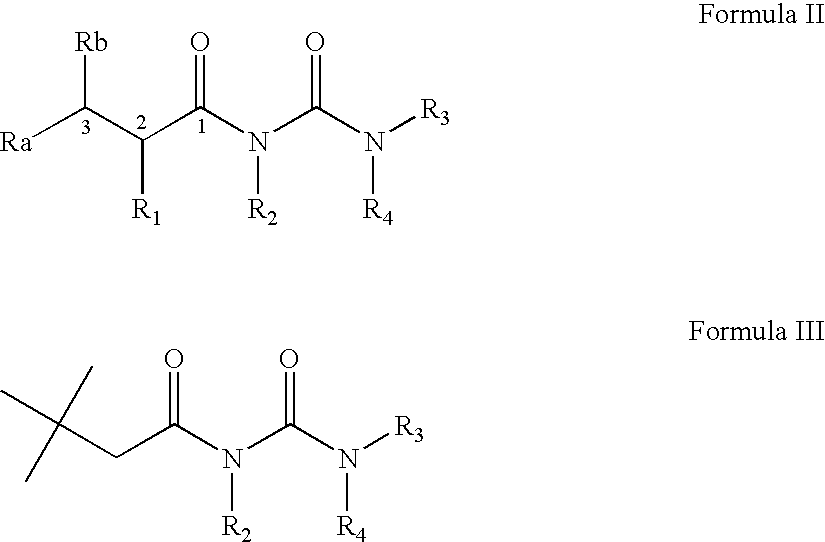
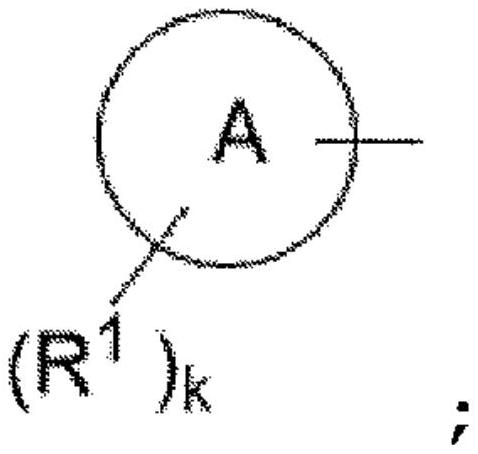
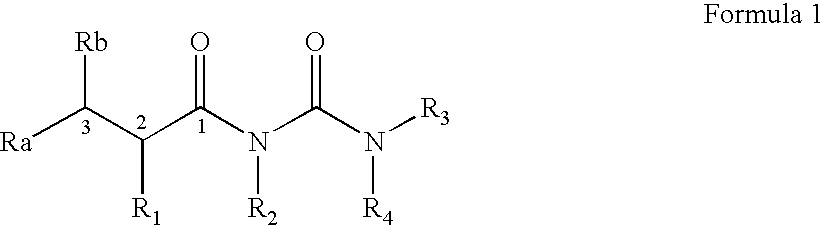
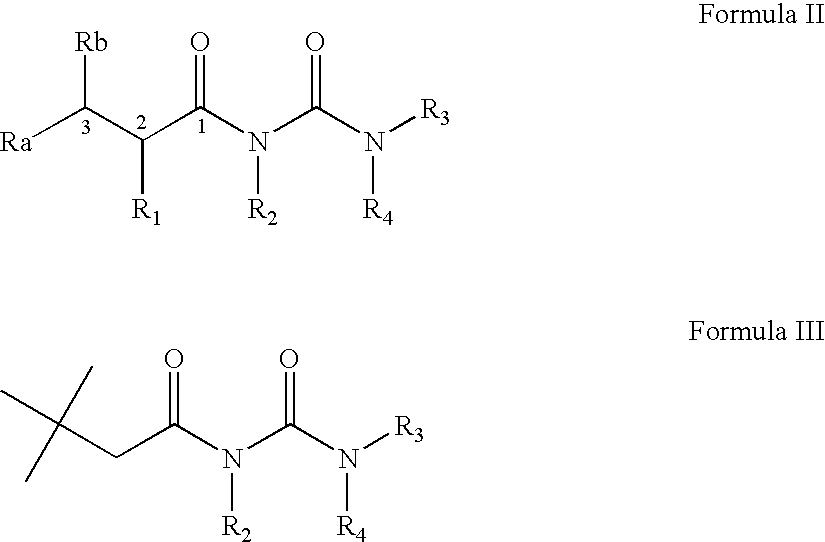
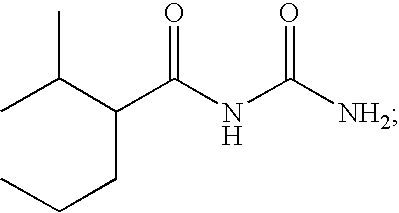
Acyl-urea derivatives and uses thereof
Novel acyl-urea containing compounds, processes of preparing same, compositions containing same and uses thereof in the treatment of neurological diseases and disorders such as epilepsy, neuropathic pain, bipolar disorder, status epilepticus, chemically-induced convulsions and / or seizure disorders, febrile convulsions conditions, metabolic disturbances and a sustenance withdrawal conditions, are provided. Also provided are uses of these and other acyl-urea containing compounds in the treatment of neurological diseases and disorders.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
A formulation for improving seizure control
PendingUS20220133652A1Improving seizure controlSeizure control has improvedNervous disorderHydroxy compound active ingredientsGeneral anaesthesiaEpileptic encephalopathy
Described herein is a method of improving seizure control in a patient experiencing uncontrolled seizures persisting 10 minutes or more, comprising administering fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, at a dose of from 0.2 to 1.2 m / kg / day for a period of about 12 hours to about 7 days to a patient having been put into a therapeutic, medically-induced coma via a general anesthetic; and after about 12 hours to about 7 days, weaning the patient from the general anesthetic and assessing whether the seizure control has improved as compared to a pre-treatment time point. The patient experiencing seizures may have epilepsy or epileptic encephalopathy that has led to established status epilepticus (SE), refractory status epilepticus (RSE) or super-refractory status epilepticus (SRSE).
Owner:ZOGENIX INT
Treatment of Prolonged Status Epilepticus
Methods for terminating, preventing, and / or treating status epilepticus are generally provided. The method can include administering a prophylactically or therapeutically effective amount of stiripentol or a related compound thereof to an individual in need of treatment of status epilepticus. The individual can be in need of treatment of prolonged status epilepticus, refractory status epilepticus, and / or benzodiazepine-resistant status epilepticus.
Owner:BICODEX +1
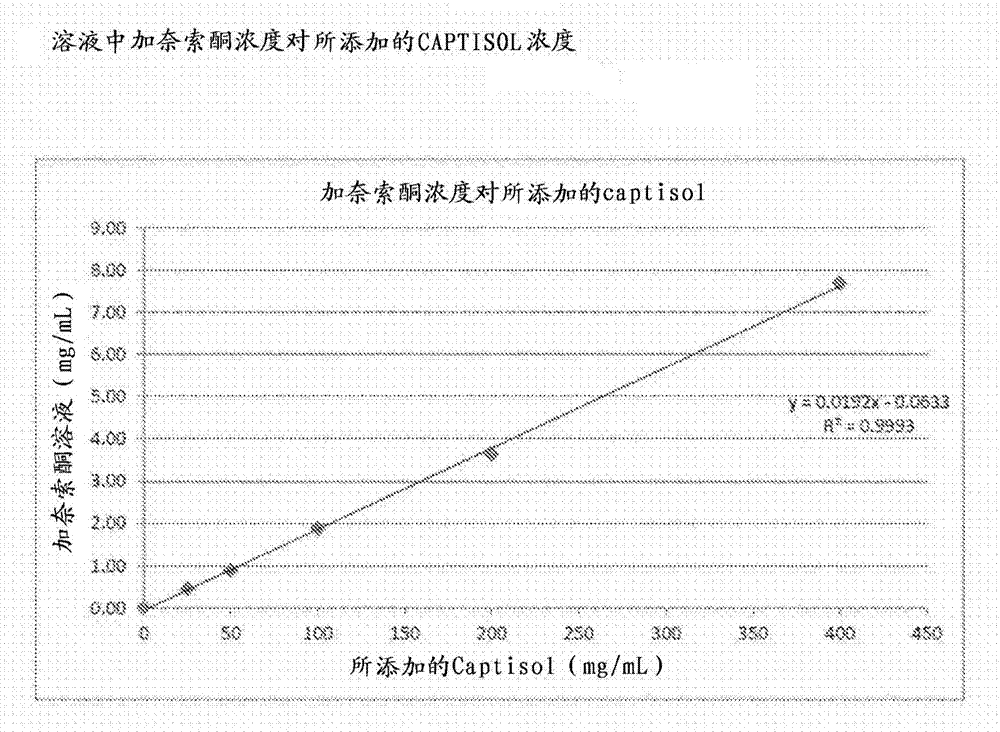
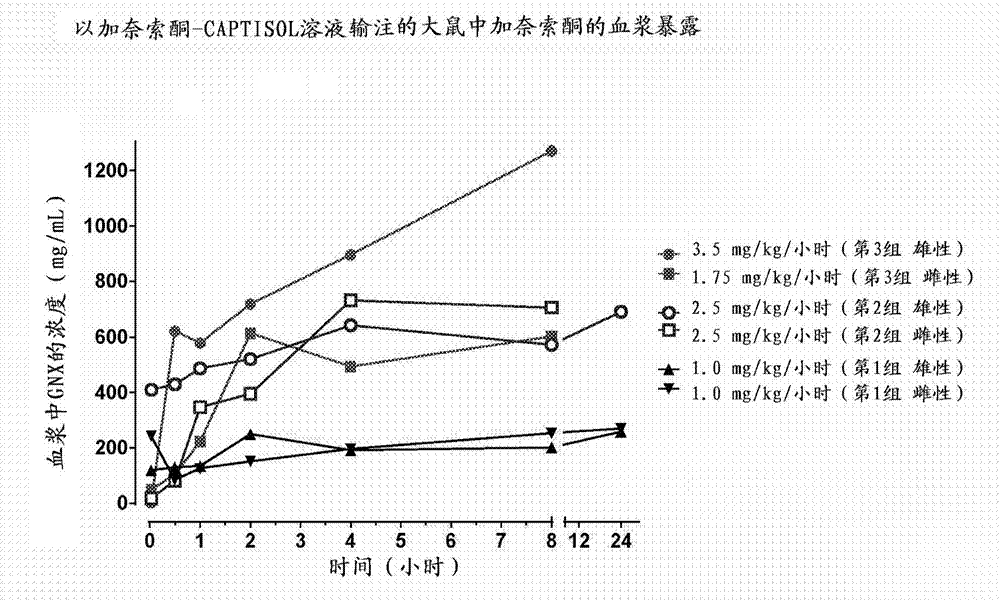
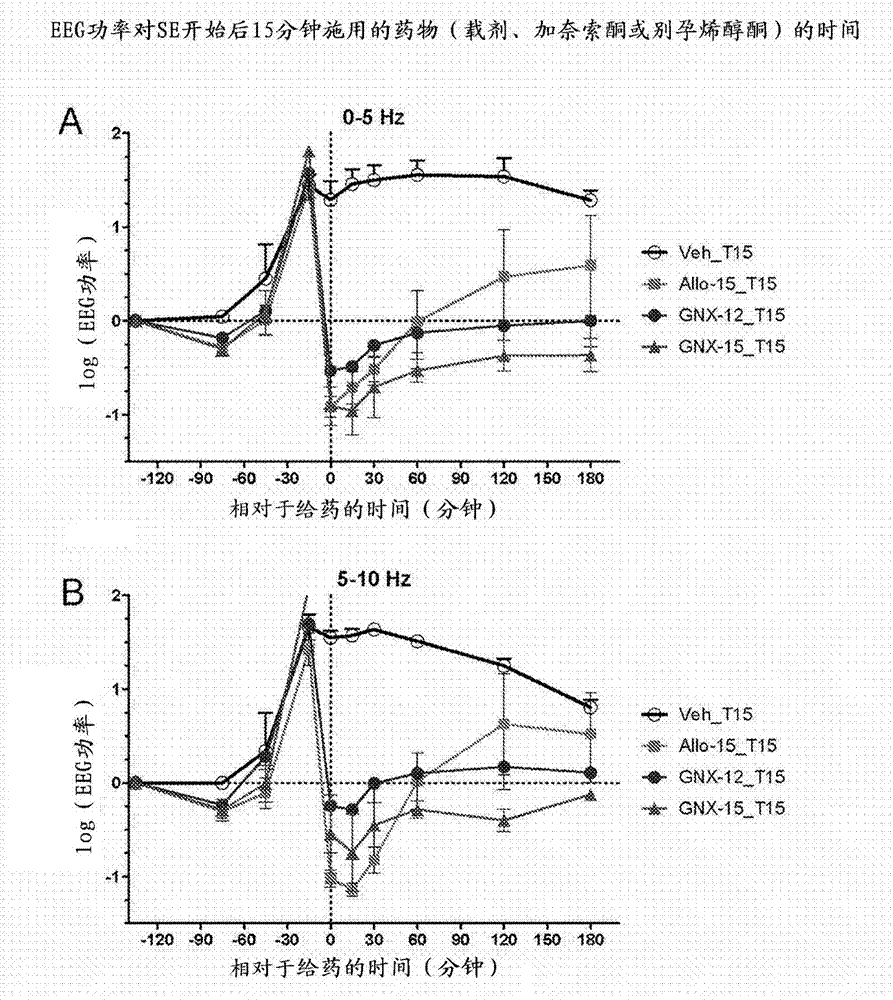
Intravenous ganaxolone formulations and their use in treating status epilepticus and other seizure disorders
The disclosure provides an injectable ganaxolone formulation comprising ganaxolone, sulfobutyl ether-beta-cyclodextrin; and water. The injectable ganaxolone formulation optionally includes a surfactant and a pH modifier. The ganaxolone and sulfobutyl ether-beta-cyclodextrin may be in an inclusion complex. The disclosure also provides a lyophilized powder of the ganaxolone / sulfobutyl ether-beta-cyclodextrin formulation that may be reconstituted in water for injection. The disclosure provides a method of treating a patient having a seizure disorder, stroke, or traumatic brain injury, comprising administering an effective amount of the injectable ganaxolone formulation comprising ganaxolone, sulfobutyl ether-beta-cyclodextrin; and water. The disclosure also provides combination methods in which the injectable ganaxolone / sulfobutyl ether-beta-cyclodextrin formulation is administered in combination with at least one additional active agent.
Owner:MARINUS PHARMA
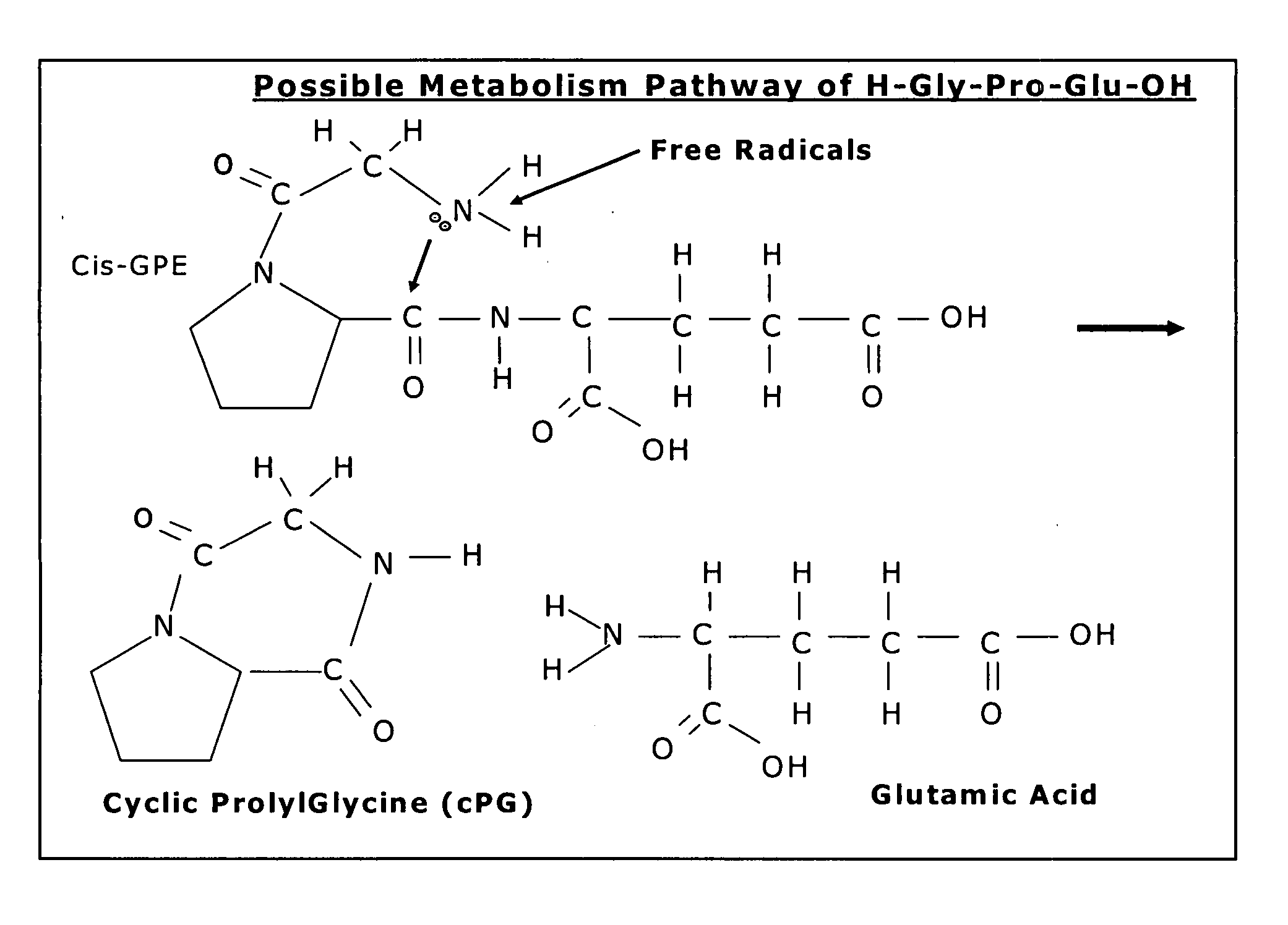
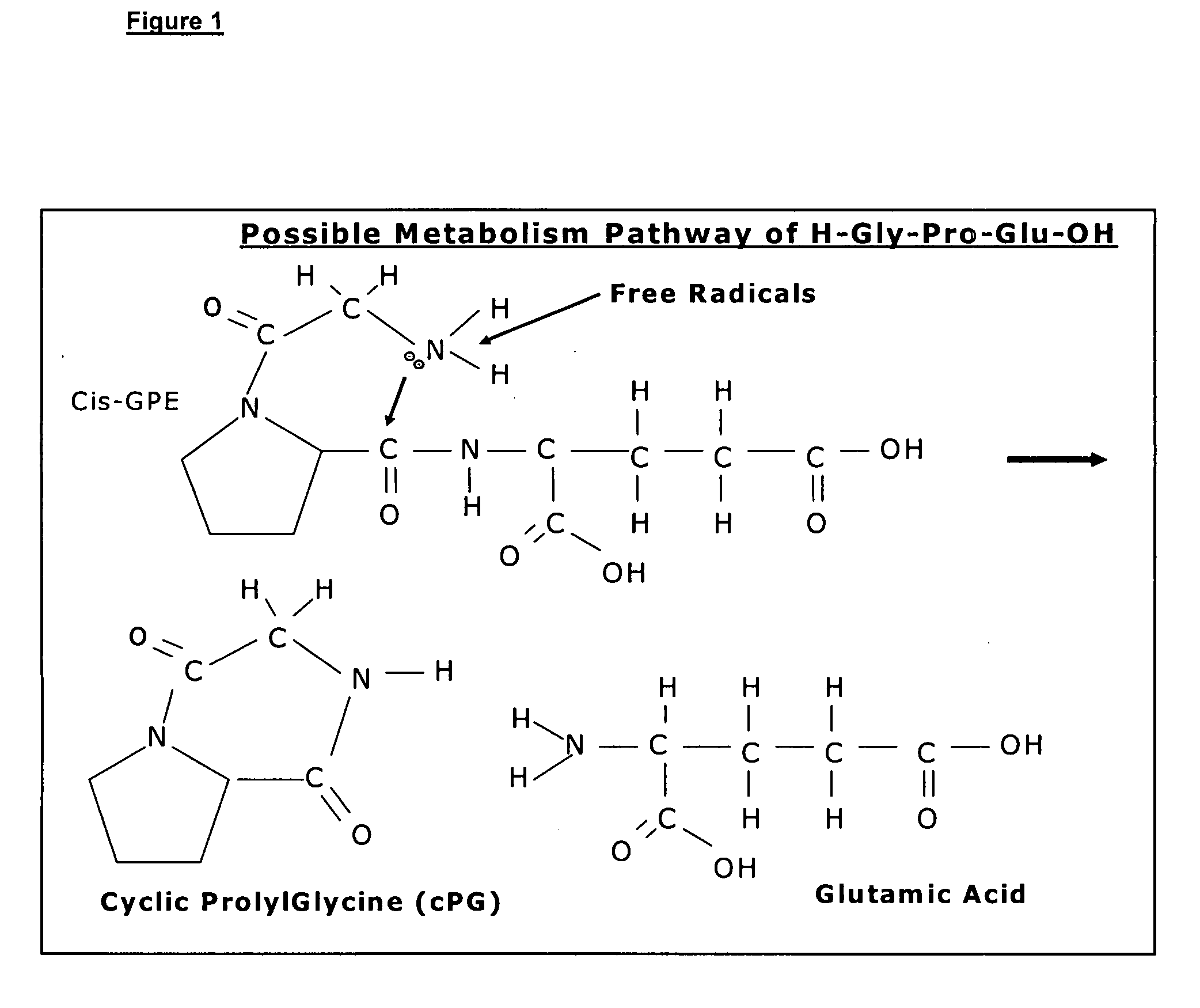
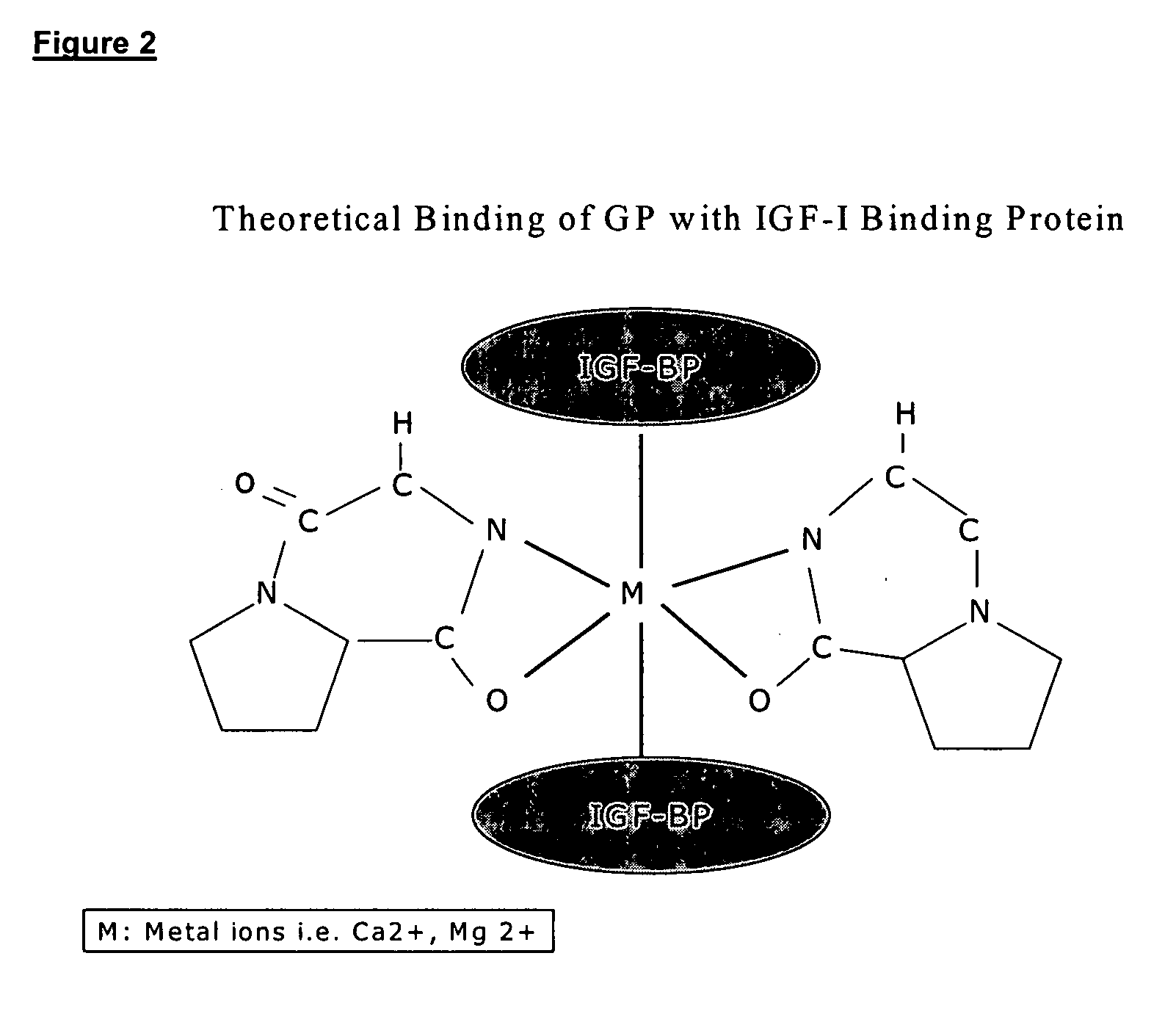
Therapeutic agent composition and method of use
The invention relates to the use of cyclic Prolyl Glycine (“cyclic PG” or “cPG”) and analogs and mimetics thereof, as neuroprotective agents for the treatment and or prevention of neurological disorders including but not limited to cerebral ischemia or cerebral infarction resulting from a range of phenomena, such as thromboembolic or hemorrhagic stroke, cerebral basospasms, hypoglycemia, cardiac arrest, status epilepticus, perinatal asphyxia, anoxia such as from drowning, pulmonary surgery, and cerebral trauma, as well as to the treatment and prevention of chronic neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease, and as anticonvulsants.
Owner:TRAN LOI H
Substituted imidazole formate type derivative and application thereof
The invention discloses a compound shown as a formula I or a stereoisomer thereof or pharmaceutically acceptable salts thereof or a solvate thereof or a prodrug thereof or a metabolic product thereofor a deuterated derivative thereof. The compound is a substituted imidazole formate type derivative with a novel structure and belongs to the field of pharmaceutical chemistry. The invention further discloses application of the substituted imidazole formate type derivative to preparation of drugs with calming, hypnosis and / or anesthetic effects and application of the substituted imidazole formatetype derivative to preparation of drugs capable of controlling status epilepticus. The compound disclosed by the invention has a relatively good inhibition effect on a central nervous system and provides a new choice for clinically screening and / or preparing the drugs with the calming, hypnosis and / or anesthetic effects and capable of controlling the status epilepticus. The formula I is shown in the description.
Owner:CHENGDU MFS PHARMA CO LTD
Substituted pyrrolecarboxylate derivative and application thereof
ActiveCN110003188ANovel structureEnhanced inhibitory effectOrganic active ingredientsNervous disorderMetaboliteSedation
The invention discloses a compound shown in a formula I, or a stereoisomer, or a pharmaceutically acceptable salt, or a solvate, or a prodrug, or a metabolite, or a deuterated derivative of the compound, and belongs to the field of medicinal chemistry. The compound is a substituted imidazolecarboxylate derivative with novel structure. The invention further discloses application of the substitutedimidazolecarboxylate derivative in preparation of drugs with effects of sedation, hypnosis and / or anesthesia and application of the substituted imidazolecarboxylate derivative in preparation of drugscapable of controlling status epilepticus, wherein the compound has good inhibition effects on the central nervous system, and a new choice is provided for clinically screening and / or preparing drugswith effects of sedation, hypnosis and / or anesthesia and drugs for controlling the status epilepticus.
Owner:CHENGDU MFS PHARMA CO LTD
Method for screening for compounds as potential sedatives or anxiolytics
InactiveUS20060013770A1Easy to measureOrganic active ingredientsNervous disorderMuscle relaxationPhysiology
The present invention relates to a method for screening a chemical compound for its potential as a sedative or anxiolytica. The invention also relates to a drug development method and to the use of a compound as identified by the screening method for the treatment, prevention or alleviation of anxiety, for inducing anaesthesia, pre-anaesthesia, muscle relaxation, or sedation, or for treatment, prevention or alleviation of fever cramps or status epilepticus in a subject.
Owner:NEUROSEARCH AS
Peptide compounds for treating refractory status epilepticus
The present invention is directed to a pharmaceutical composition comprising a Compound (a) of a class of peptide Compounds and at least one further Compound (b) for the prevention, alleviation or / and treatment of epileptic seizures.
Owner:UCB SA
Application of roseolic acid C to preparation of medicine for preventing and/or treating epilepsy
ActiveCN111658630ANervous disorderAnhydride/acid/halide active ingredientsPharmaceutical medicineAbnormal EEG
The invention relates to an application of roseolic acid C or pharmaceutically acceptable salts or hydrates thereof of therapeutically effective amount to preparation of a medicine for preventing and / or treating epilepsy for a testee having requirements, wherein the roseolic acid C can restore abnormal EEG during status epilepticus, can reduce epileptic attack rate and attack level, can restrain activity of hippocampus excitatory neurotransmitter receptors NMDA, can agitate activity of hippocampus inhibiting neurotransmitter receptors GABA, reduce cerebral cortex excitability, further restrainepileptic formation and exert the epileptic resisting effect.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
Composition and use thereof in enhancing a therapeutic effect of an antiepileptic drug
InactiveUS20090156565A1Good treatment effectReduces progressive resistanceBiocideNervous disorderAntiepileptic drugTherapeutic effect
A composition and a method for the use of the composition for enhancing a therapeutic effect of an antiepileptic drug in treating a seizure in a status epilepticus in an animal. A composition includes a glucocorticoid receptor antagonist and an antiepileptic drug, wherein the glucocorticoid receptor antagonist is present in an amount effective to enhance a therapeutic effect of the antiepileptic drug in treating a seizure in a status-epilepticus in an animal. A method of use of a glucocorticoid receptor antagonist for enhancing a therapeutic effect of an antiepileptic drug in treating a seizure in a status epilepticus in an animal, the method includes administering the glucocorticoid receptor antagonist and the antiepileptic drug, wherein the glucocorticoid receptor antagonist is administered to the animal prior to, contemporaneous with, or subsequent to administering the antiepileptic drug in an amount effective to enhance the therapeutic effect of the antiepileptic drug.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
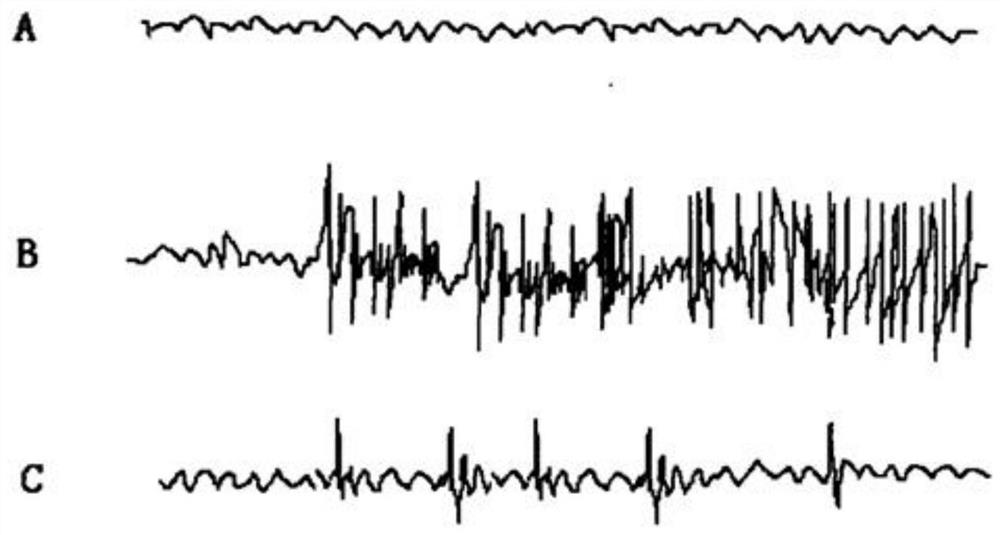
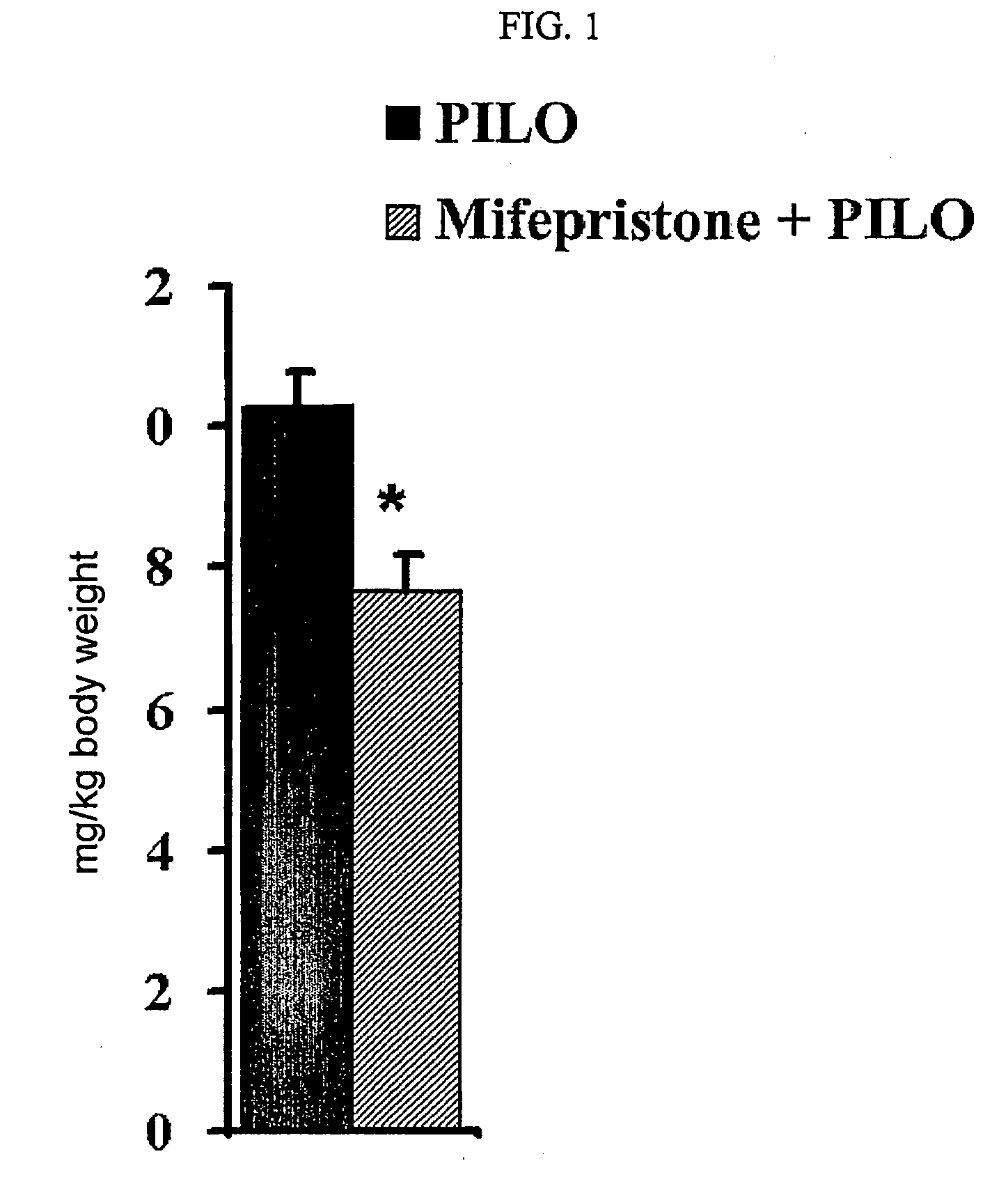
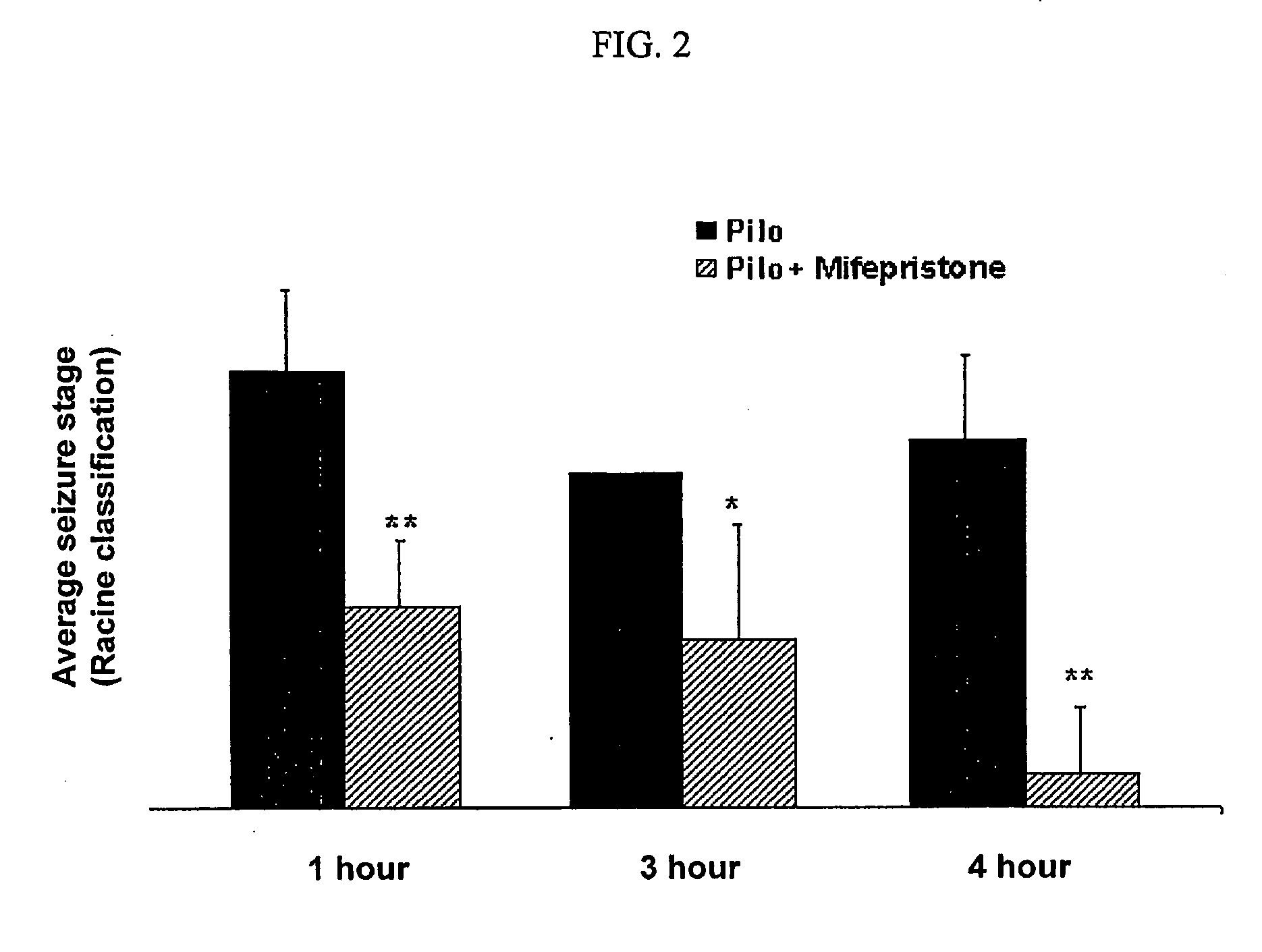
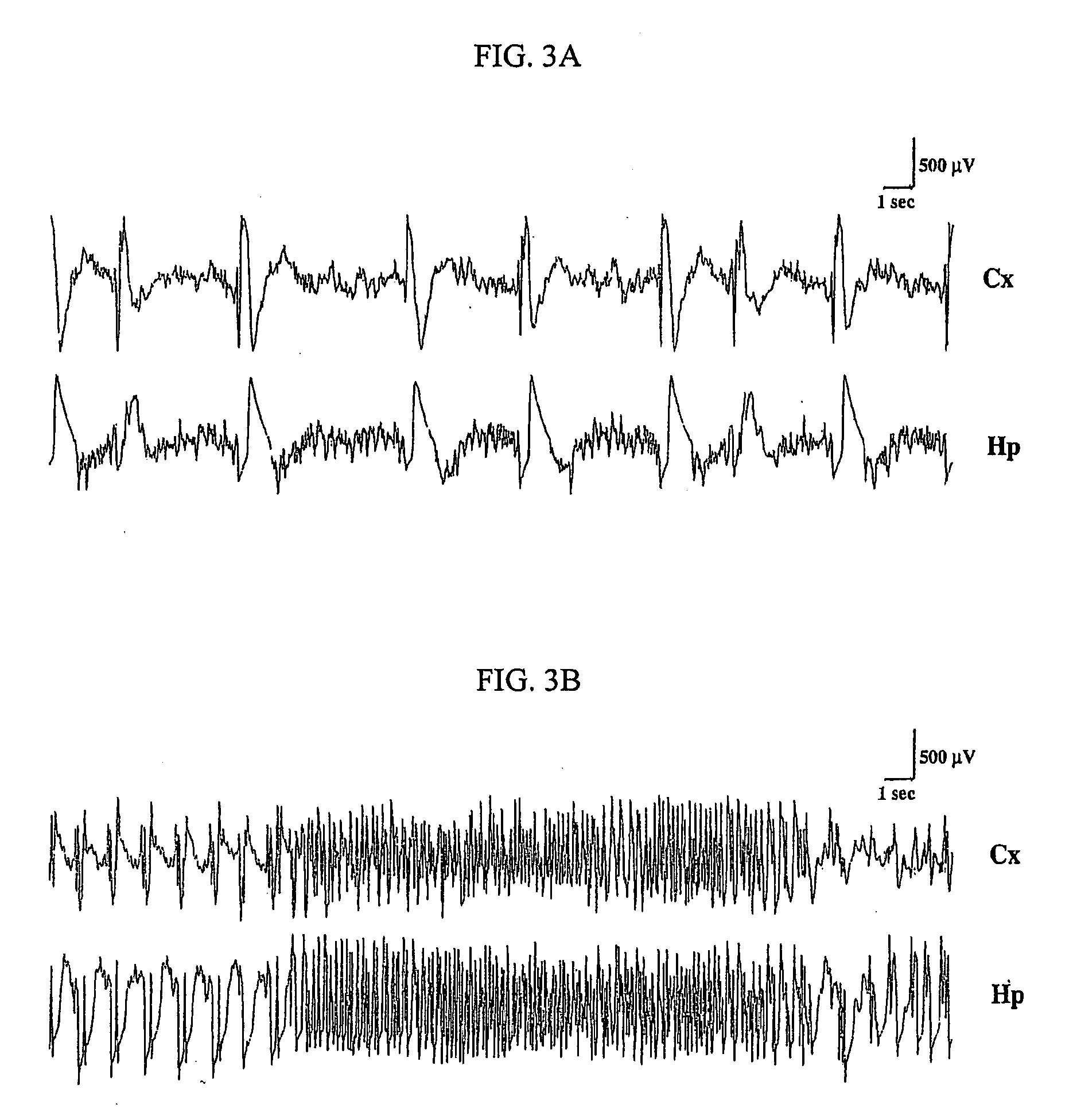
Application of pb in the preparation of drugs for preventing or treating pilo-induced epilepsy
ActiveCN112675183BExtended incubation periodShorten duration of stateOrganic active ingredientsNervous disorderPharmaceutical drugDrugs preparations
The invention belongs to the field of pharmaceutical preparations, and in particular relates to the application of barisenoside B (PB) in the preparation of drugs for preventing or treating epilepsy induced by pilocarpine (PILO). For the first time, it was found that barisenoside B can significantly prolong the latency period of pilocarpine-induced epilepsy, shorten the duration of status epilepticus, reduce the grade of epileptic seizures, and slow down the weight loss caused by epilepsy, so it has a good effect on PILO-induced epilepsy Preventive and therapeutic effects. It has important clinical application value in pilocarpine-induced epilepsy, and at the same time opens up a new drug use for the active ingredient of barisonoside B.
Owner:THE AFFILIATED HOSPITAL OF SHANDONG UNIV OF TCM
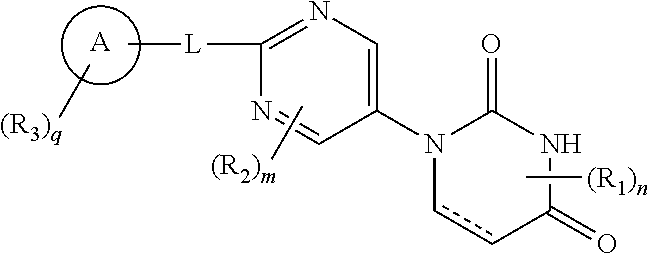
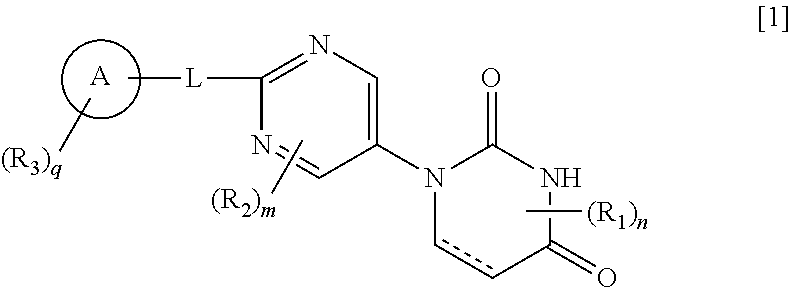
Pyrimidine compound
ActiveUS10800758B2Effective preventingEffective diagnosingOrganic active ingredientsSilicon organic compoundsDiseaseSymptomatic seizures
The present invention provides a novel pyrimidine compound represented by Formula [I] and a salt thereof:[in the formula, the symbols are as defined in the specification], which is useful for treating, preventing and / or diagnosing seizure and the like in disease involving epileptic seizure or convulsive seizure (including multiple drug resistant seizure, refractory seizure, acute symptomatic seizure, febrile seizure and status epilepticus), as well as a medical use therefor.
Owner:OTSUKA PHARM CO LTD
Heterocyclic compounds for the treatment of epilepsy
PendingCN113166076AEffective treatmentLittle side effectsOrganic active ingredientsNervous disorderDiseaseSymptomatic seizures
Owner:OTSUKA PHARM CO LTD
Amino acid derivatives of substituted quinexaline 2,3-dione derivatives as glutamate receptor antagonists
A novel series of substituted quinoxaline 2,3-diones useful as neuroprotective agents are taught. Novel intermediates, processes of preparation, and pharmaceutical compositions containing the compounds are also taught. The compounds are glutamate antagonists and are useful in the treatment of stroke, cerebral ischemia, or cerebral infarction resulting from thromboembolic or hemorrhagic stroke, cerebral vasospasms, hypoglycemia, cardiac arrest, status epilepticus, perinatal asphyxia, anoxia, Alzheimer's, Parkinson's, and Huntington's diseases.
Owner:WARNER-LAMBERT CO
Acyl-urea derivatives and uses thereof
Novel acyl-urea containing compounds, processes of preparing same, compositions containing same and uses thereof in the treatment of neurological diseases and disorders such as epilepsy, neuropathic pain, bipolar disorder, status epilepticus, chemically-induced convulsions and / or seizure disorders, febrile convulsions conditions, metabolic disturbances and a sustenance withdrawal conditions, are provided. Also provided are uses of these and other acyl-urea containing compounds in the treatment of neurological diseases and disorders.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
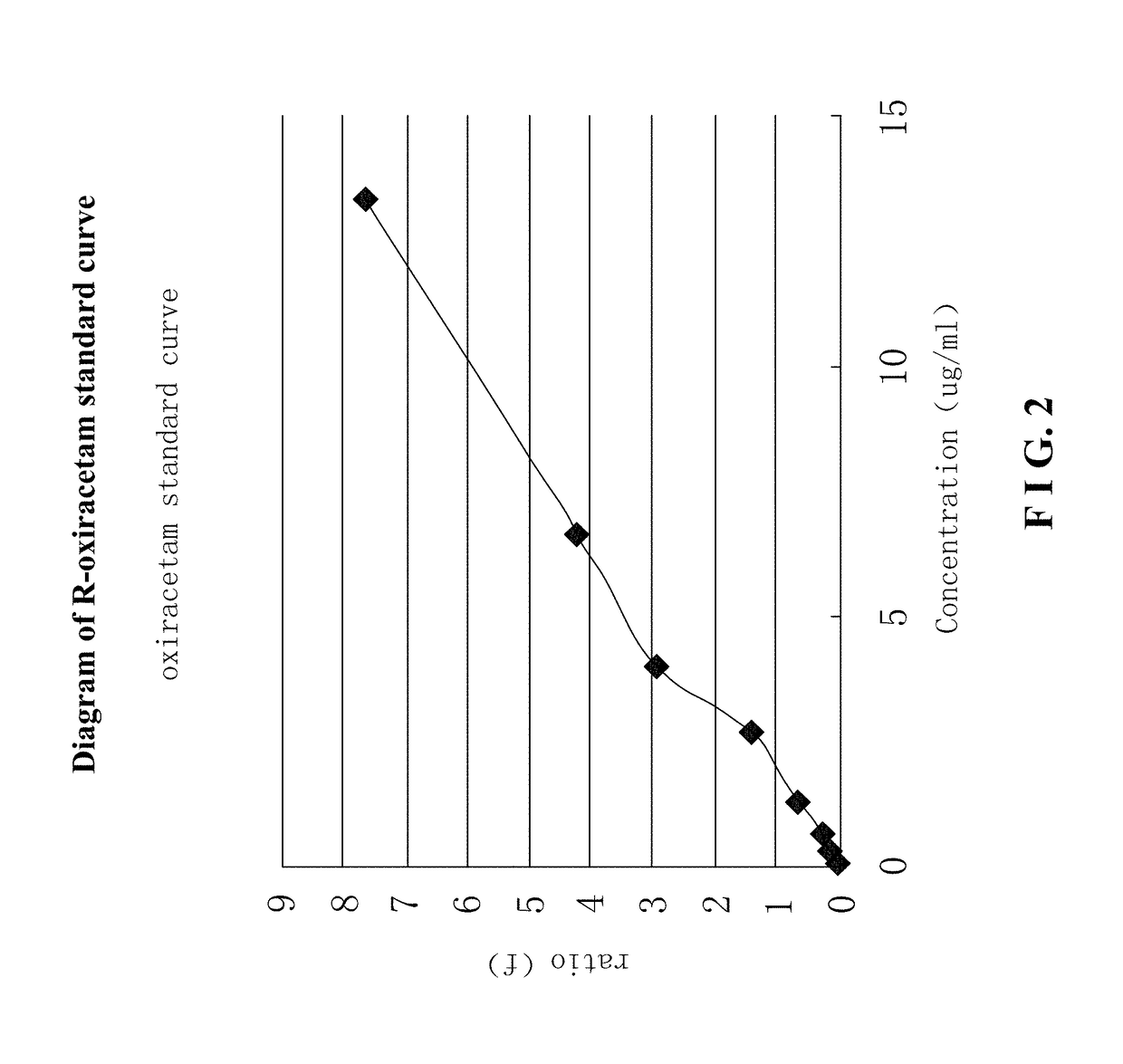
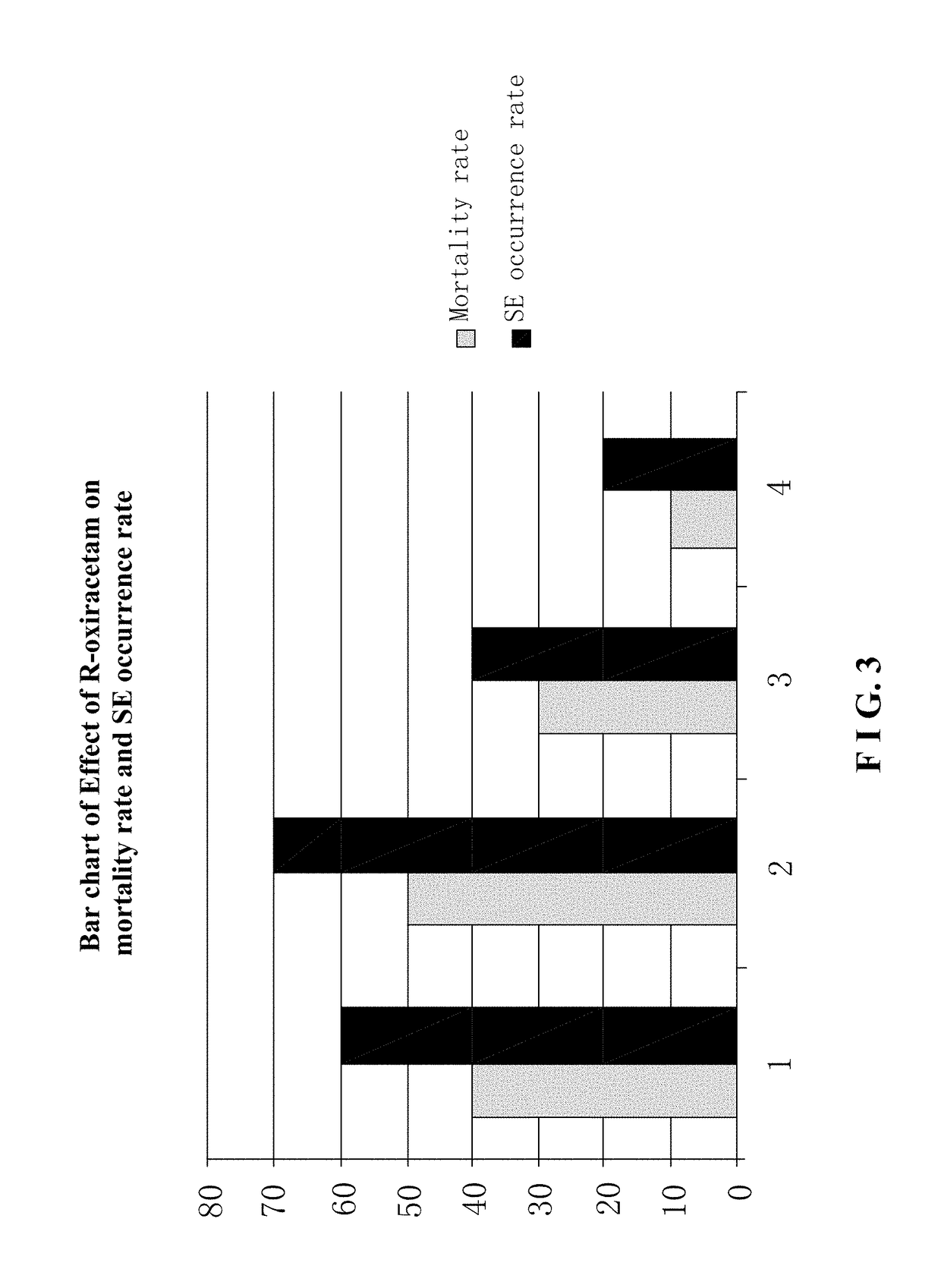
Use of r-oxiracetam in pharmaceutical field
InactiveUS20180147183A1Accurate weighingEnhanced inhibitory effectOrganic active ingredientsNervous disorderPartial epilepsyEpilepsy seizure
Provided is a use of R-oxiracetam in the preparation of a drug for preventing or treating epilepsy. An experimental result shows that the R-oxiracetam has an obvious effect in the treatment of generalized epilepsy seizure, partial epilepsy seizure and status epilepticus.
Owner:CHONGQING RUNZE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com