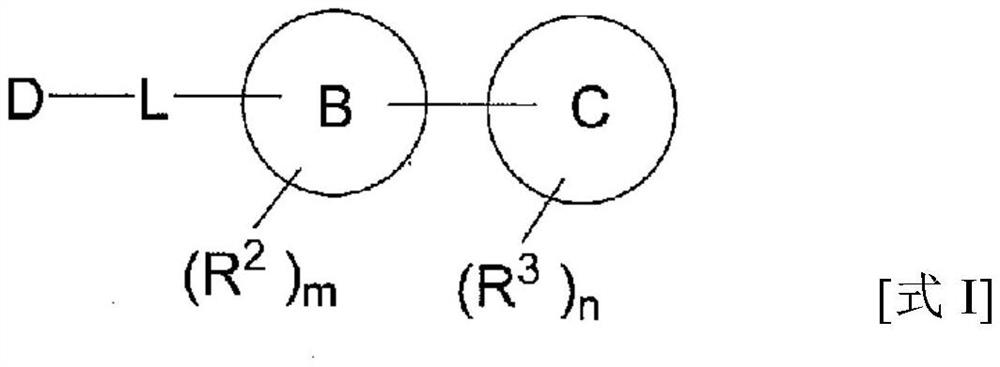
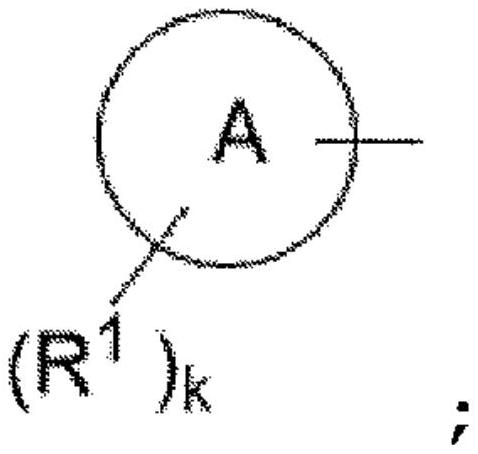
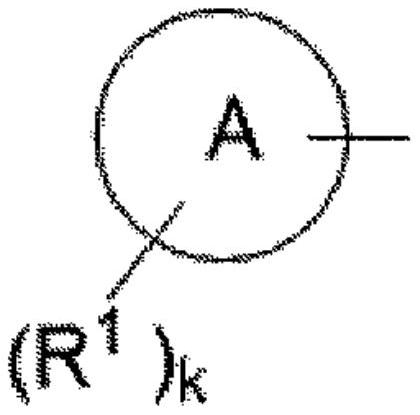Heterocyclic compounds for the treatment of epilepsy
A compound and heterocyclic technology, applied in the field of heterocyclic compounds and their salts, can solve the problems of epilepsy, motor dysfunction, disturbing the balance of neuronal activity, etc., and achieve the effect of wide therapeutic spectrum and few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0396] Hereinafter, the present invention is further described in detail by the following test examples, reference examples and examples, but they do not limit the present invention, and they can be changed to the extent that they do not depart from the scope of the present invention.
[0397] The following abbreviations are used in this specification.
[0398]
[0399]
[0400]
[0401]
[0402] In the examples below, "room temperature" generally indicates about 10°C to about 35°C. Ratios indicated for mixed solvents are by volume unless otherwise indicated. Percentages indicate % by weight unless otherwise indicated.
[0403] 1 HNMR (proton nuclear magnetic resonance spectrum) was measured by Fourier transform type NMR (either of Bruker AVANCE III 400 (400 MHz) and Bruker AVANCE III HD (500 MHz)). In silica gel column chromatography, when expressed as basic, aminopropylsilane-bonded silica gel is used.
[0404] The absolute configuration of the compound was a...
Embodiment 1
[0527] Synthesis of 3-methoxy-6-(2-phenoxypyrimidin-5-yl)pyridazine
[0528] Containing 2-phenoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine (5.00g), 3-chloro -6-methoxypyridazine (3.64g), PdCl 2 (dppf)DCM (0.137g), K 3 PO 4 (7.12g), 1,4-di A mixture of alkanes (50 mL) and water (25 mL) was heated to reflux for 2 hours under nitrogen atmosphere. The reaction solution was poured into water, and the product was extracted with AcOEt. The organic layer was washed with water and brine and dried over anhydrous sodium sulfate, then concentrated. The residue was purified by silica gel column chromatography (DCM / AcOEt) to obtain the title compound (4.42 g).
Embodiment 4
[0530] Synthesis of 3-(2-(3-fluorophenoxy)pyrimidin-5-yl)-6-methoxypyridazine
[0531] Will contain 5-bromo-2-(3-fluorophenoxy)pyrimidine (2.234g), (BPin) 2 (2.63g), PdCl 2 (dppf)DCM (0.282g), AcOK (1.358g) and 1,4-di The mixture of alkanes (20 mL) was heated to reflux for 2 hours under a nitrogen atmosphere. To the reaction solution was added 3-chloro-6-methoxypyridazine (1.00 g), PdCl 2 (dppf)DCM (0.282g), K 3 PO 4 (2.94 g) and water (5 mL), and the mixture was heated to reflux overnight under an atmosphere of nitrogen. Water and AcOEt were added to the reaction solution, the mixture was filtered through celite, and the product was extracted with AcOEt. The organic layer was washed with water and brine and dried over anhydrous sodium sulfate, then concentrated. The precipitated crystals were washed with EtOH, so as to obtain the target compound (947 mg).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com