Immunochemotherapy pharmaceutical composition and preparation method therefor
A technology for chemoimmune and chemotherapeutic drugs, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as damage to normal organs, high toxicity and side effects of chemotherapy, and low clinical response rates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1: Preparation and use of sodium alginate (the first type of component) and imiquimod (the third type of component) hydrochloride composition freeze-dried powder injection
[0136] Step 1: Preparation of imiquimod (the third component) hydrochloride. Weigh 50-100 mg of imiquimod into a 50 ml glass mixing container, add 1 ml of 1M dilute hydrochloric acid therein, and add deionized water to dilute after the white powdery imiquimod is fully dissolved until it is colorless and transparent, so that The final concentration of imiquimod is 2.5-5 mg / ml. The solution was freeze-dried to obtain freeze-dried powder of imiquimod hydrochloride. The purpose of this step is to convert the water-insoluble imiquimod into the water-soluble hydrochloride form. Sufficient lyophilization time is required to ensure complete removal of hydrochloric acid residues.
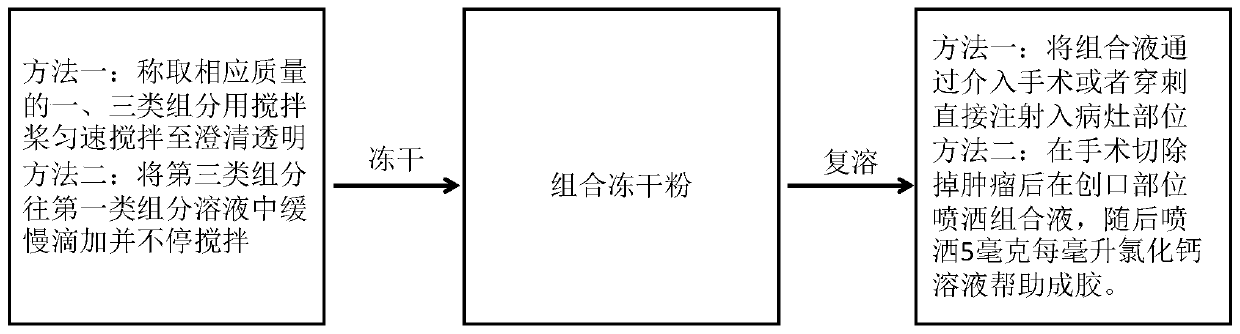
[0137] Step 2: The following three methods can be used to prepare the freeze-dried powder injection of the hydrochlor...
Embodiment 2
[0151] Example 2: Sodium alginate (the first type of component) and CpG oligonucleotide (the third type of component) composition freeze-dried powder injection
[0152] Step 1: Preparation of sodium alginate and CpG oligonucleotide composition freeze-dried powder injection
[0153] Weighing 10-80 mg of sodium alginate and 0.1-5 mg of CpG oligonucleotides are dissolved in 1 ml of aqueous phase solution, fully shaken until the solution is clear and transparent, and then freeze-drying the solution to obtain a freeze-dried powder injection of the composition.
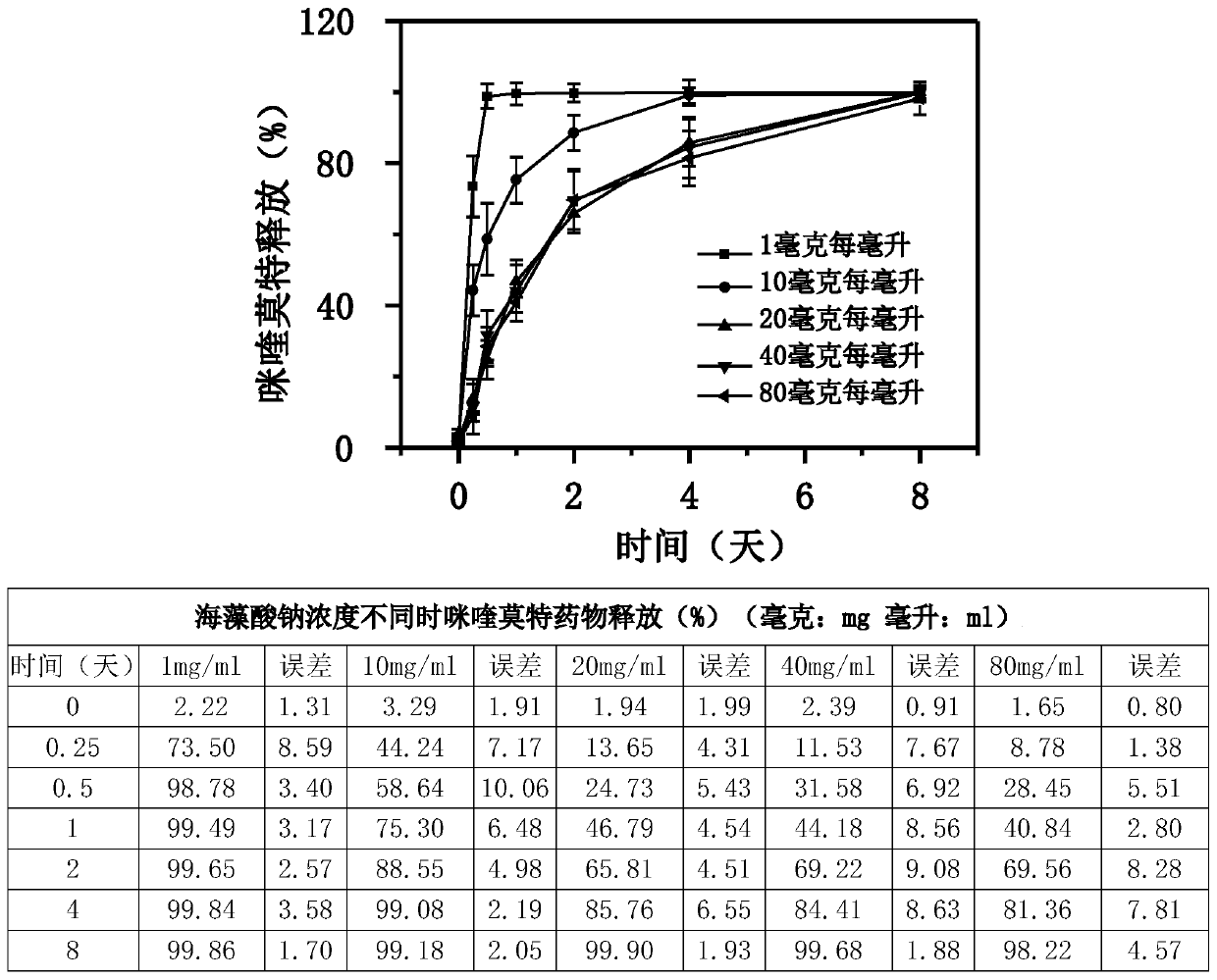
[0154] Figure 5 It is a scanning electron microscope picture of the freeze-dried powder injection of the composition after reconstitution into a gel. It can be seen from the figure that the composition still has good gelation ability after freeze-drying and reconstitution, and from the electron microscope pictures, it can be seen that there are many micron-scale pores after gelation, which is of great help to the sustaine...
Embodiment 3
[0163] Embodiment three: Sodium alginate (the first type of component) and doxorubicin hydrochloride (the second type of component) composition freeze-dried powder injection
[0164] Step 1: Preparation of freeze-dried powder injection of sodium alginate and doxorubicin hydrochloride composition:
[0165] Method 1: Weigh 20-80 mg of sodium alginate and 0.1-10 mg of doxorubicin hydrochloride and dissolve them in 1 ml of aqueous phase solution, stir with a stirring paddle at a speed of 50-300 rpm until the solution is clear and transparent, and then dissolve The solution is freeze-dried to obtain the composition freeze-dried powder injection.
[0166] Method 2: Dissolve 0.1-10 mg of doxorubicin hydrochloride in 1 ml of aqueous phase solution, stir with a stirring paddle at a speed of 50-300 rpm until the solution is clear and transparent, and then dissolve 10-80 mg of sodium alginate Into the aqueous phase solution, add the constantly stirring doxorubicin hydrochloride solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com