Application of linker of polyethylene glycol and fat-soluble compounds in biological catalysis
A fat-soluble compound, polyethylene glycol technology, which is applied in the application field of linker in biocatalysis, can solve the problems of substrate solubility and cell/enzyme activity and stability being difficult to take into account at the same time, so as to improve the reaction rate. And the effect of separation and purification efficiency, improving transformation efficiency, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 3-oxooleanolic acid-28-(4-methylpolyethylene glycol carboxylate)-benzyl ester
[0045]
[0046] Synthetic route of 3-oxooleanolic acid-28-(polyethylene glycol monoester 4-carboxylate)-benzyl ester
[0047] (1) Preparation of 4-bromomethylbenzoyl chloride: add 4-bromomethyl-benzoic acid (2.15g 10mmol) and toluene (10ml) in a flask, slowly add oxalyl chloride (2.97ml 35mml) dropwise, and stir at room temperature 10min, heated and stirred at 50°C for 3h. After the reaction was completed, it was evaporated to dryness under reduced pressure for later use.
[0048] (2) Preparation of 4-bromomethylbenzoic acid polyethylene glycol monoester: add mPEG (5g 2.5mmol) and toluene (20ml), and reflux for 24h. After the reaction was completed, the toluene was evaporated to dryness under reduced pressure to obtain a yellow oily solid, which was washed three times with 20 ml of anhydrous ether, filtered, and dried in vacuo to obtain a white powder (4.97 g 2.27 mmol yi...
Embodiment 2
[0052] Preparation of 3-methylpolyethylene glycol succinyl oleanolic acid
[0053]
[0054] Synthetic route of 3-methylpolyethylene glycol succinyl oleanolic acid
[0055] (1) Synthesis of 1,4-succinic acid methyl polyethylene glycol monoester: add m-PEG (20g 10mmol), succinic anhydride (10g 10mmol), pyridine (8ml 10mmol) and chloroform in a flask (50ml), reflux for 48h. After the reaction was completed, the chloroform was evaporated to dryness under reduced pressure, and the precipitate was dissolved in a saturated sodium bicarbonate solution (200ml) in an ice bath, and the insoluble matter was filtered off, extracted twice with ethyl acetate (20ml) (removing pyridine), and the filtrate was cooled to Acidify with hydrochloric acid at 0-5°C, adjust PH = 2, extract 3 times with dichloromethane (100ml), dry the organic layer with anhydrous sodium sulfate, evaporate dichloromethane to dryness under reduced pressure to obtain an oily solid, wash with anhydrous diethyl ether (1...
Embodiment 3
[0060] Synthesis of 3-(1-methylpolyethylene glycol monoester-4-succinoyl)-cholesterol
[0061]
[0062] Synthetic route of 3-(1-methylpolyethylene glycol monoester-4-succinyl)-cholesterol
[0063] (1) Synthesis of 3-(1-methylpolyethylene glycol monoester-4-succinyl)-cholesterol: add m-PEG-DA (4.2g2mmol), cholesterol (3.87g 10mmol) and three Methyl chloride (10ml), oxalyl chloride (0.425mmol) was slowly added dropwise, stirred at room temperature for 10min, and heated to reflux for 10h. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure.
[0064] (2) The precipitate was washed three times with saturated sodium bicarbonate solution (100ml), then washed twice with anhydrous ether (100ml), filtered,
[0065] (3) The obtained crude product was purified by column chromatography (dichloromethane:petroleum ether=1:3), and dried in vacuo to obtain a light yellow solid. (3.45g1.4mmol yield 70%)
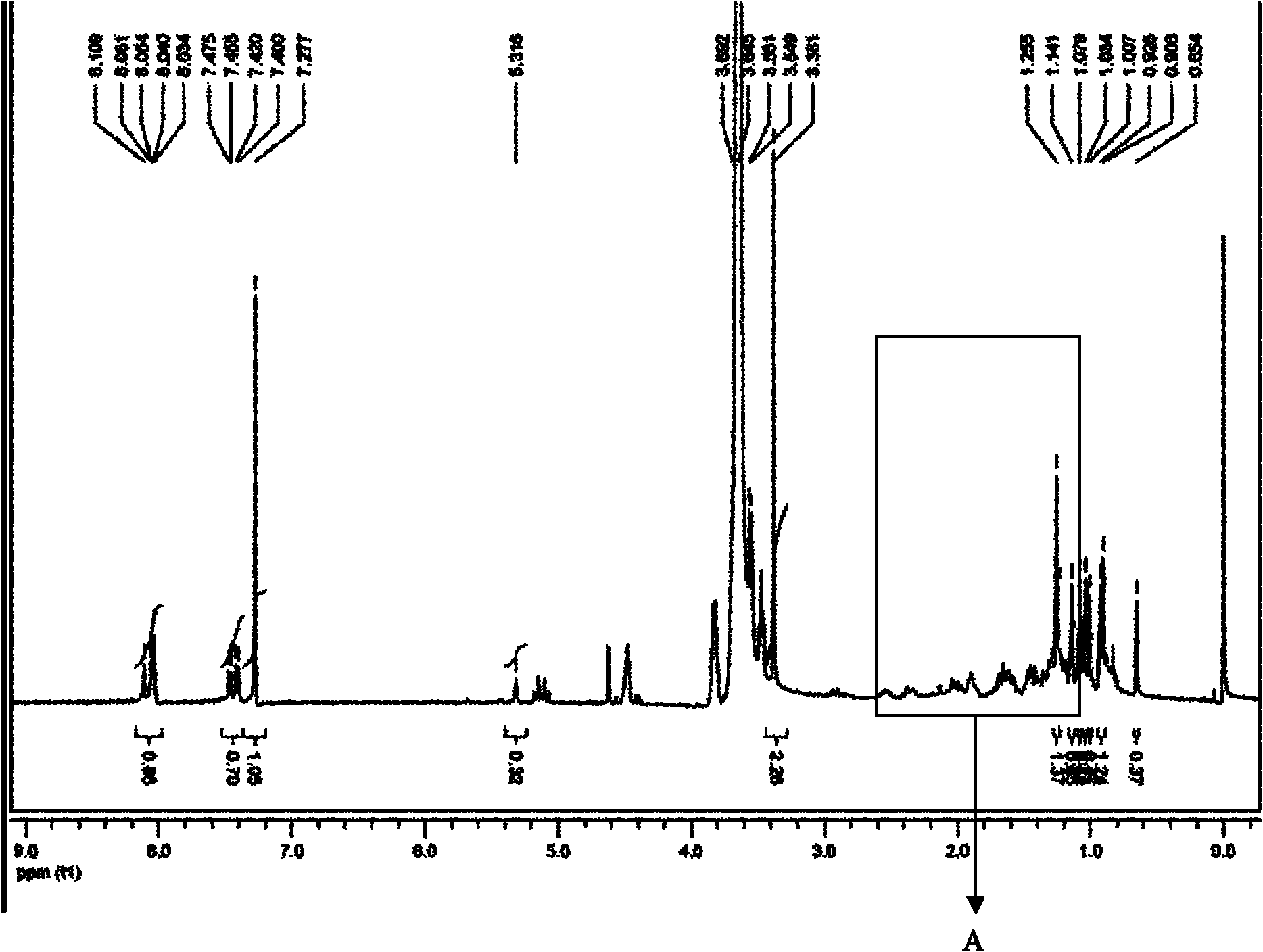
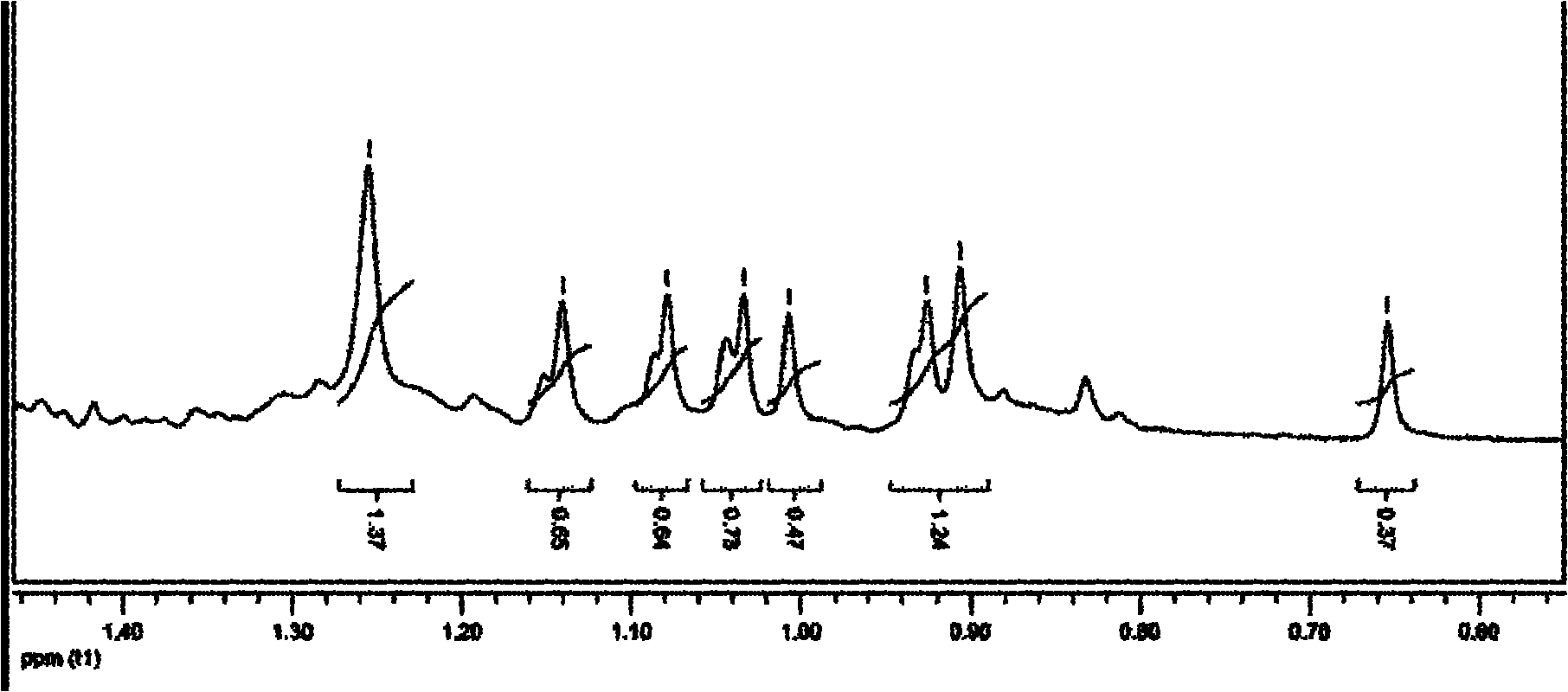
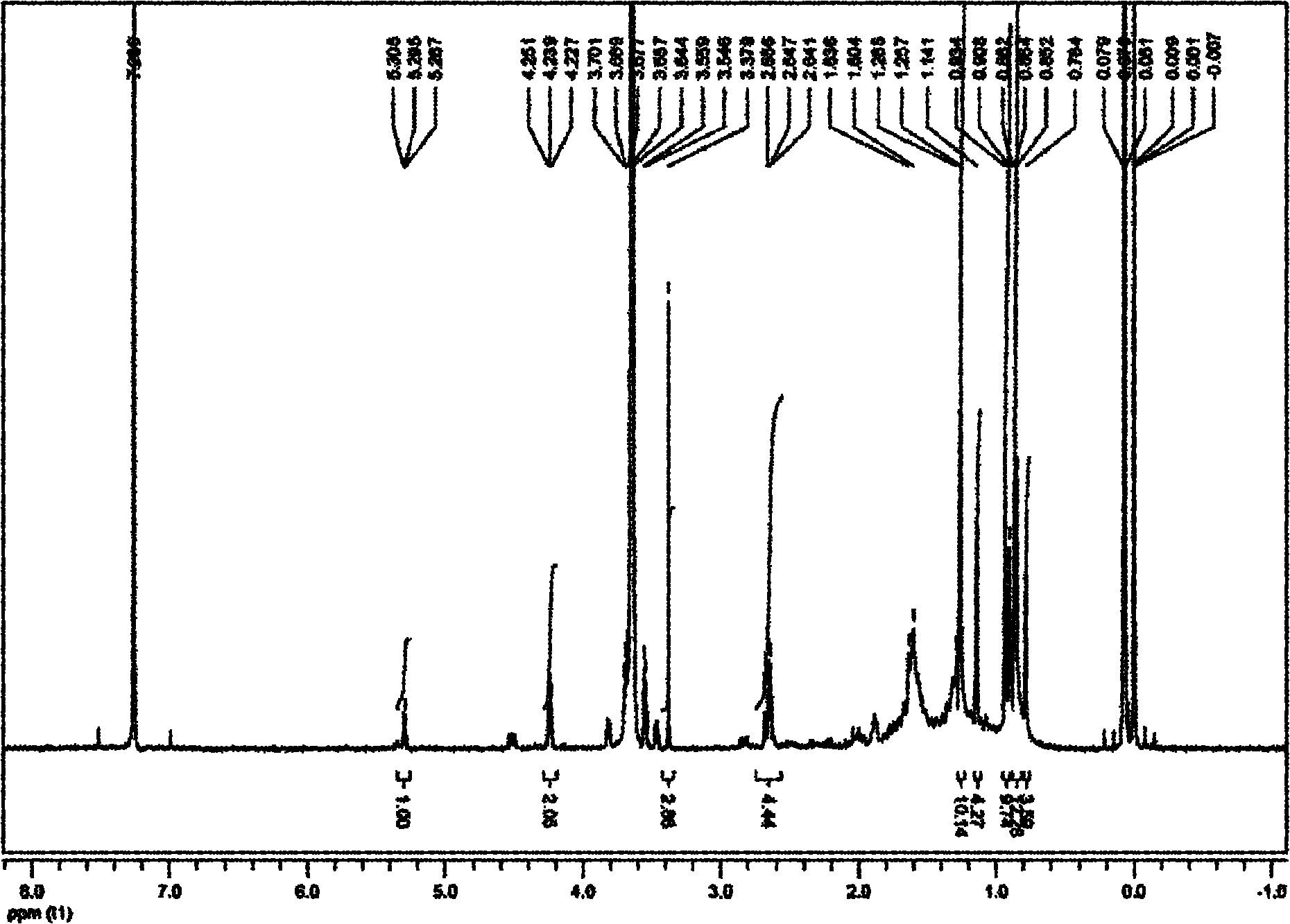
[0066] 1 HNMR (CDCl 3 , 400MHz): 2.64(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com