Water soluble ethopabate composition and compound composition thereof
A technology of ethoxyn amidine and compound composition, which is applied in the field of water-soluble ethoxyn amidine compositions and compound compositions thereof, and can solve the problems such as difficult mixing of premixes, affecting treatment effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Pulverization of ethopabate
[0026] Crushing equipment: QYF-2600 jet mill Kunshan Miyou Machinery Manufacturing Co., Ltd.
[0027] Crushed sample source: ethopabate (Fuyang Chengxing Chemical Auxiliary Co., Ltd. batch number: 20120302)
[0028] Set the pressure of the air compressor to 0.4Mpa, the speed of the classifying wheel to 7200 rpm, the crushing pressure of the main air valve to 0.8MPa, and the pressure to the blowback valve to 0.4-0.6MPa. The crushing amount is 5-10g / sec. Qualified ethopabate can be obtained after crushing.
[0029] Particle size determination of ethopabate micropowder:
[0030] Determination method: "Pharmacopoeia of the People's Republic of China" appendix particle size determination method, method 1, microscope determination method.
[0031] A. Calibration of eyepiece micrometer
[0032] The size of the powder particle size is measured by the eyepiece micrometer (referred to as the eyepiece micrometer) placed in the eyepiece...
Embodiment 2
[0043] Embodiment 2: the impact of ultrafine grinding on the solubility of ethopabate
[0044] 1. Grinding of ethopabate
[0045] The ethopabate provided in the present invention is pulverized, and its particle size is ≤20 μm.
[0046] Crushing equipment: QYF-2600 jet mill Kunshan Miyou Machinery Manufacturing Co., Ltd.
[0047] Crushed sample source: ethopabate (Fuyang Chengxing Chemical Auxiliary Co., Ltd. batch number: 20120302)
[0048] Set the pressure of the air compressor to 0.4Mpa, the speed of the classifying wheel to 7200 rpm, the crushing pressure of the main air valve to 0.8MPa, and the pressure to the blowback valve to 0.4-0.6MPa. The crushing amount is 5-10g / sec. Qualified ethopabate can be obtained after crushing.
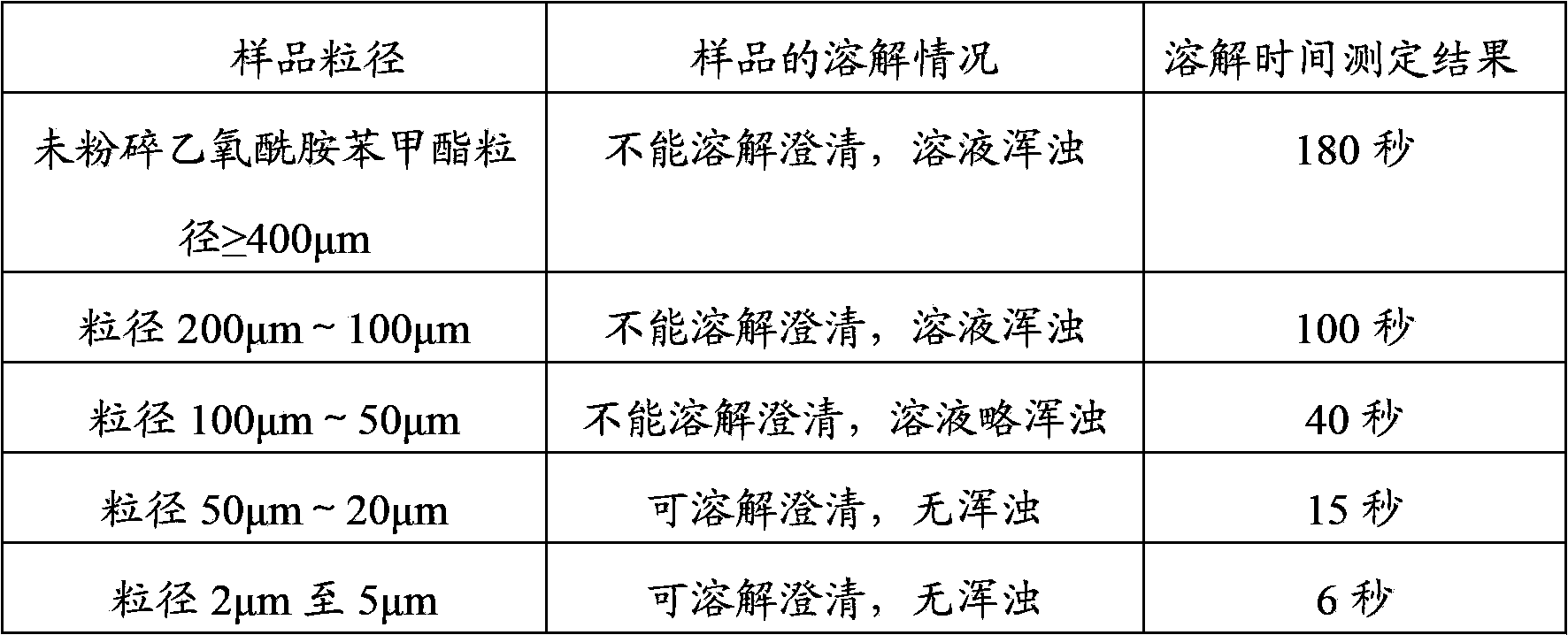
[0049] 2. Comparison of the dissolution rate of ethopabate containing different particle sizes in purified water
[0050] Weigh 0.1 g of ethopabate (Fuyang Chengxing Chemical Auxiliary Co., Ltd. batch number: 20120302) with different particle si...
Embodiment 3
[0064] Preparation of soluble compound composition containing ethopabate:
[0065]
[0066] Preparation method: Weigh 0.5 g of crushed ethopabate and 10.0 g of sodium carbonate, mix well, then add amprolium hydrochloride 10.0 g, sulfaquinoxaline sodium 6.0 g, mix well, add glucose 73.5 g and mix uniformly to obtain a soluble powder compound composition.
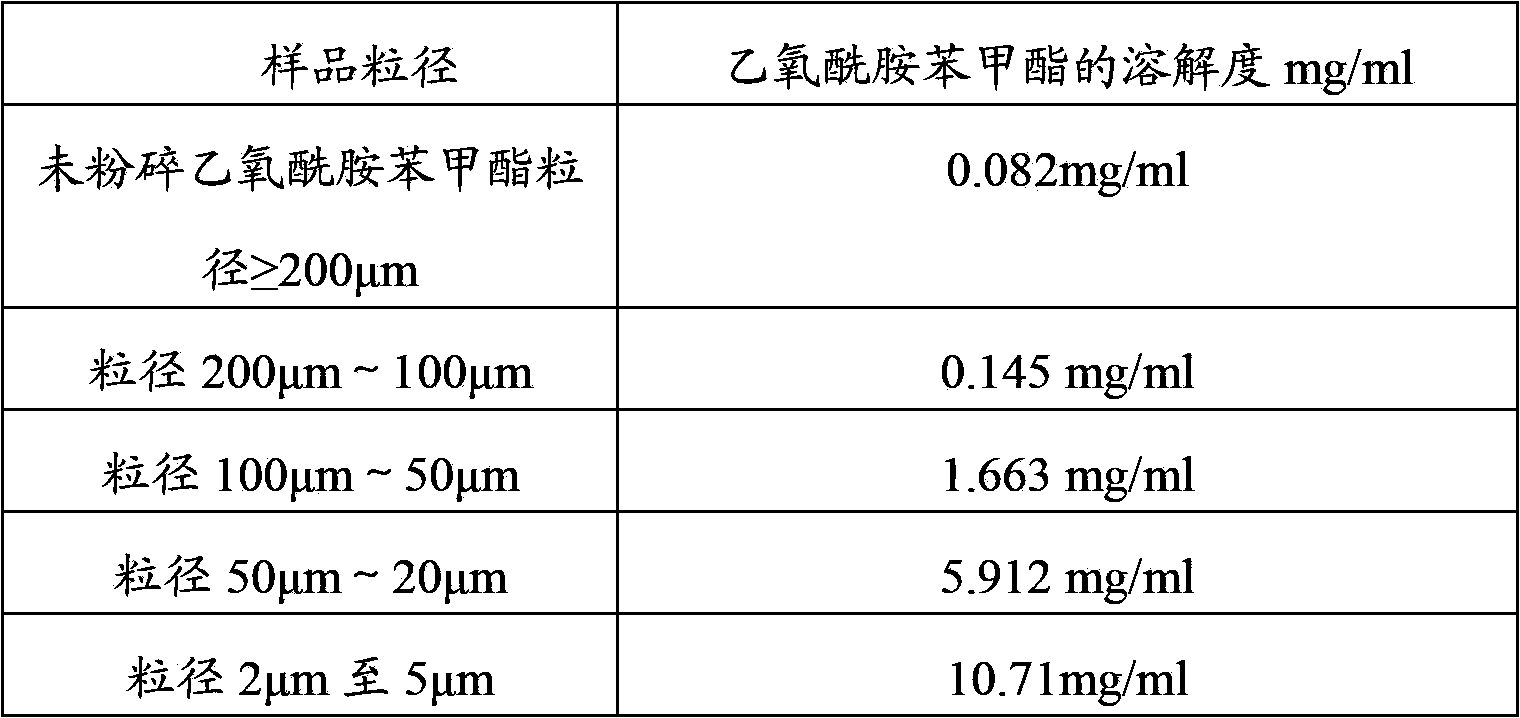
[0067] Solubility determination of ethopabate in the soluble compound composition containing ethopabate:
[0068] Take 100g of the above-prepared soluble compound composition and place it in 100ml of purified water at 25±2°C, place it at room temperature for 2 hours, filter the solution, and take the filtrate. Determination of the method under the item, the content of ethopabate in the HPLC assay solution.
[0069]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com