Method for preparing interleukin-11 semi-finished product solution, and products thereof
A technology of interleukin and semi-finished products, which is applied in the direction of peptide/protein components, medical preparations containing active ingredients, blood diseases, etc., can solve the problems of low solubility, unstable activity, and reduced clinical use, and achieve quantitative accuracy and clarity Good, the effect of reducing the amount of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
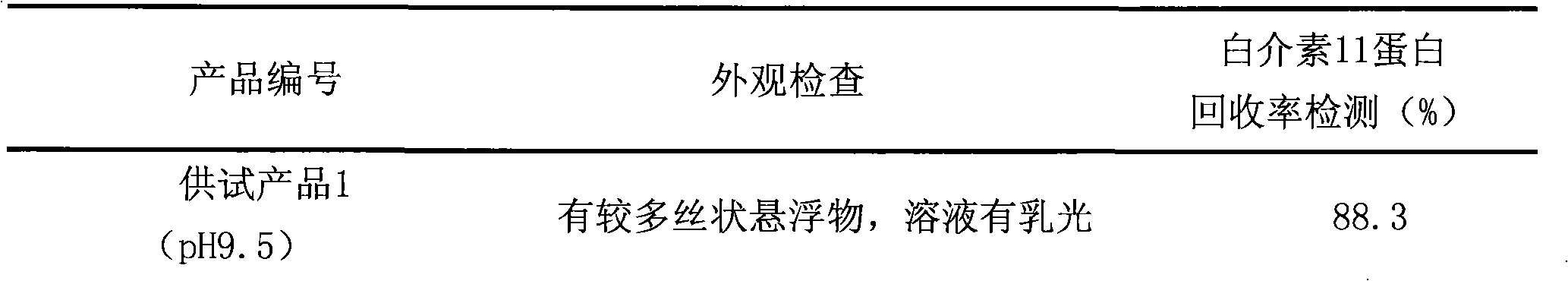
[0013] Weigh 30g of glycine (injection-grade pharmaceutical excipient) as an additive, and dissolve the measured interleukin-11 stock solution (calculated and added according to the concentration of the stock solution, the final concentration of interleukin-11 is 0.75mg / ml) in phosphate buffer solution with a final concentration of 10mM (prepared by 2.18 grams of pharmaceutical grade sodium dihydrogen phosphate dodecahydrate and 0.61 grams of disodium hydrogen phosphate dihydrate, pH7.0), stir to obtain a mixed solution; adjust the concentration of the mixed solution with sodium hydroxide of 0.1mol / L pH value to 9.5; add water for injection to 1.0L, stir and mix to obtain product 1.
Embodiment 2
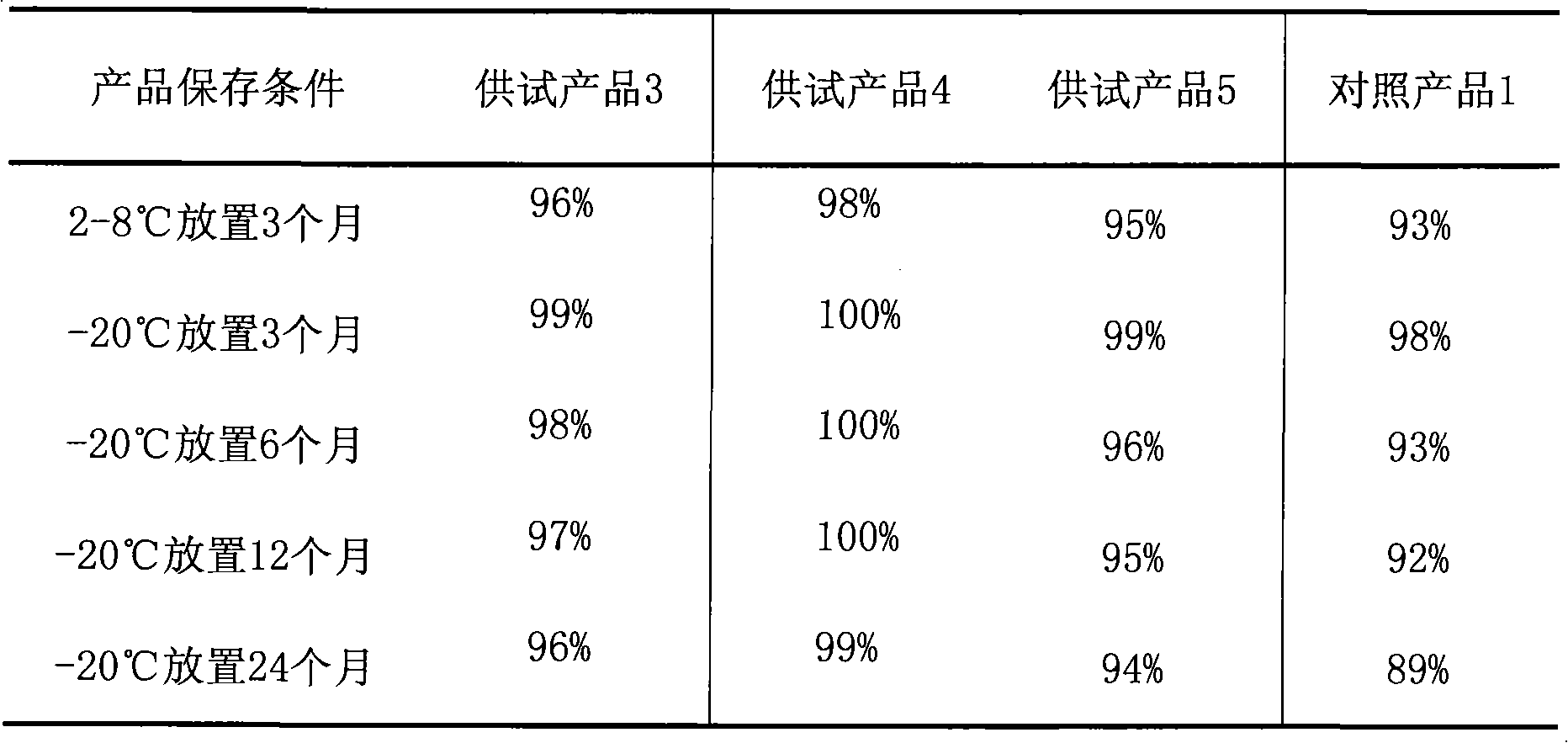
[0015] Weigh 30g of glycine (injection-grade pharmaceutical excipient) as an additive, and dissolve the measured interleukin-11 stock solution (calculated and added according to the concentration of the stock solution, the final concentration of interleukin-11 is 0.75mg / ml) in phosphate buffer solution with a final concentration of 10mM (prepared by 2.18 grams of pharmaceutical grade sodium dihydrogen phosphate dodecahydrate and 0.61 grams of disodium hydrogen phosphate dihydrate, pH7.0), stir to obtain a mixed solution; adjust the concentration of the mixed solution with sodium hydroxide of 0.1mol / L pH value to 8.0; add water for injection to 1.0L, stir and mix to obtain product 2.
Embodiment 3
[0017] Weigh 30g of glycine (injection-grade pharmaceutical excipient) as an additive, and dissolve the measured interleukin-11 stock solution (calculated and added according to the concentration of the stock solution, the final concentration of interleukin-11 is 0.75mg / ml) in phosphate buffer solution with a final concentration of 10mM (prepared from 2.18 grams of pharmaceutical grade sodium dihydrogen phosphate dodecahydrate and 0.61 grams of disodium hydrogen phosphate dihydrate, pH 7.0), stir to obtain a mixed solution; adjust the pH value of the mixed solution with 0.1mol / L hydrochloric acid to 6.0; add water for injection to 1.0L, stir and mix to obtain product 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com