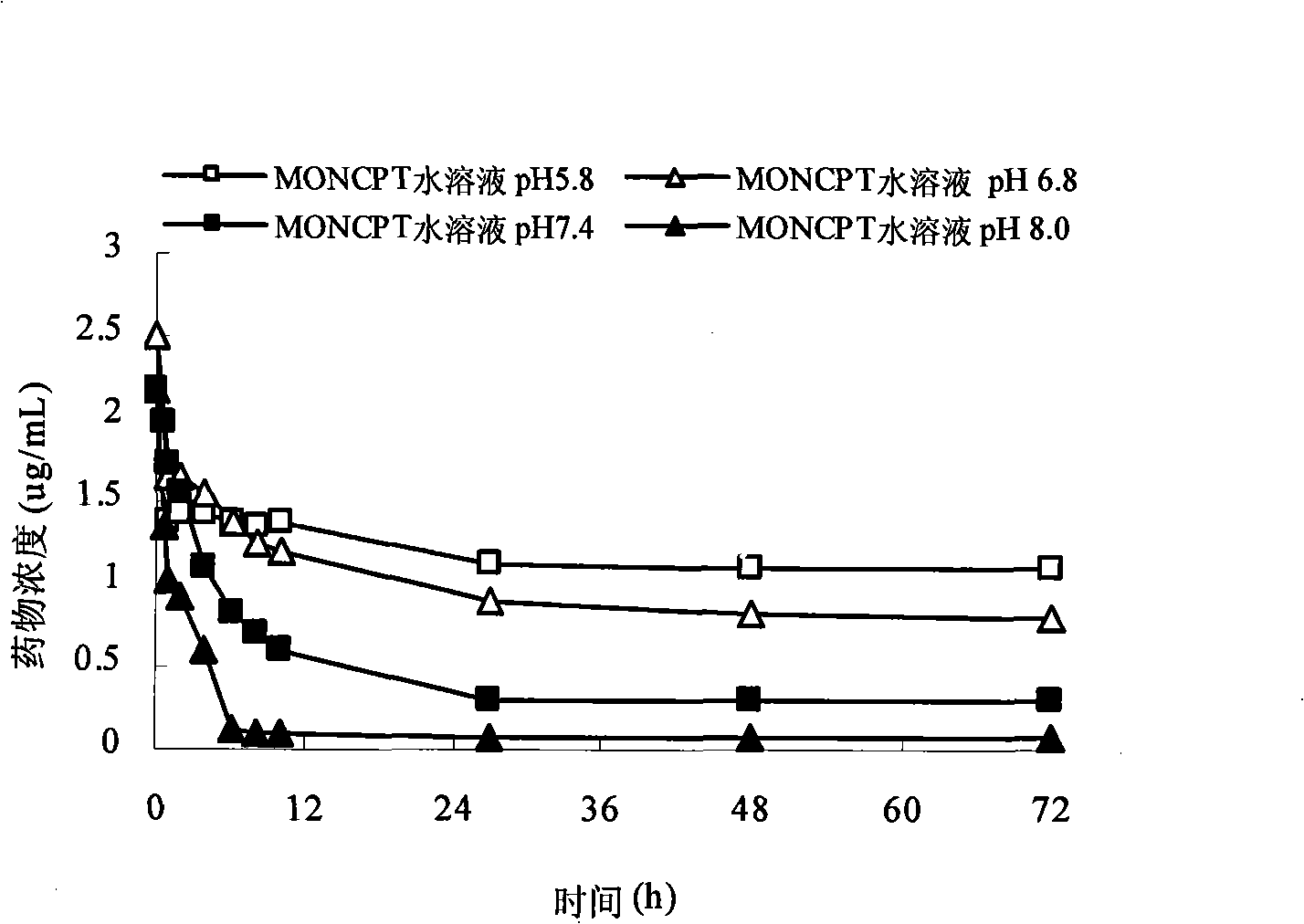
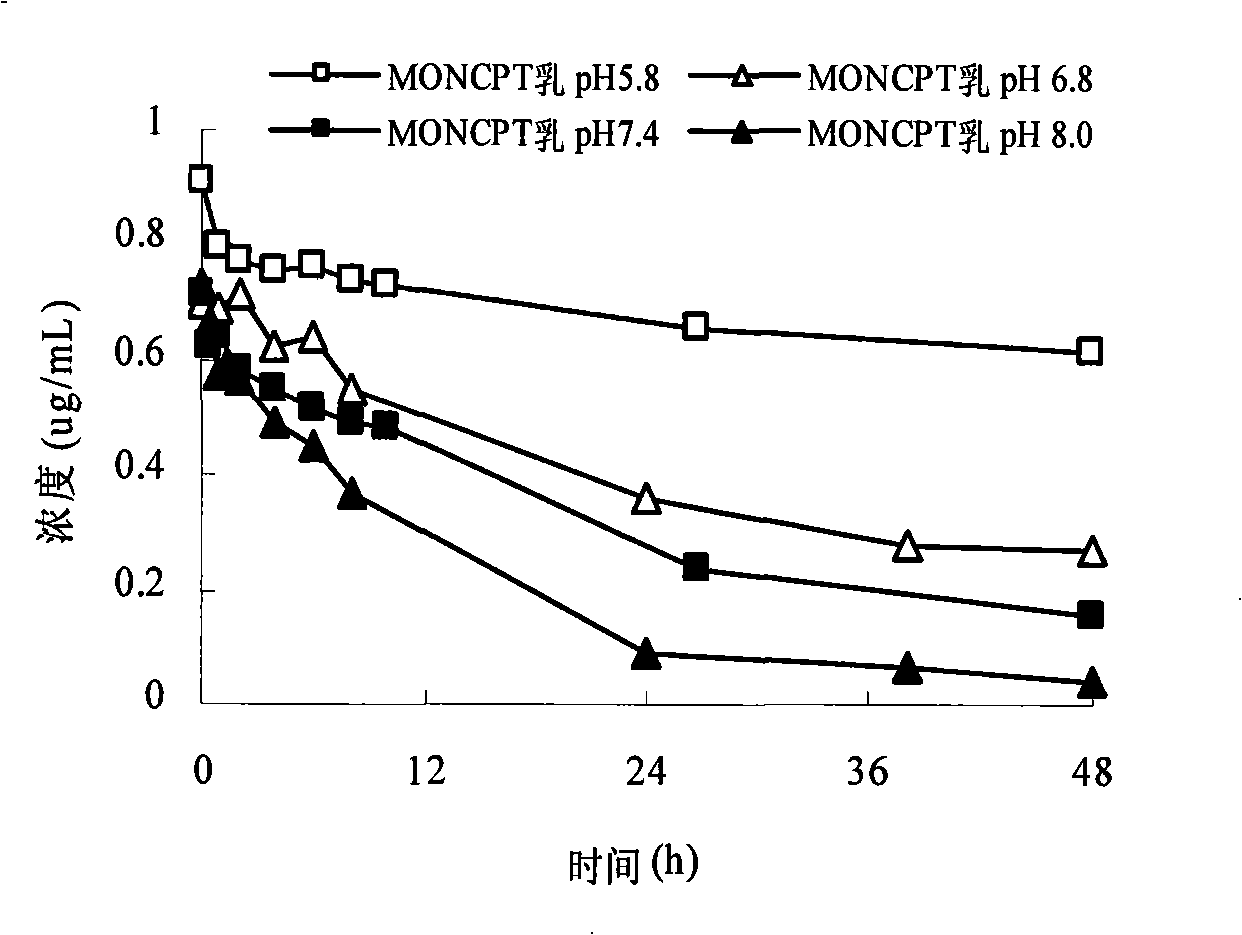
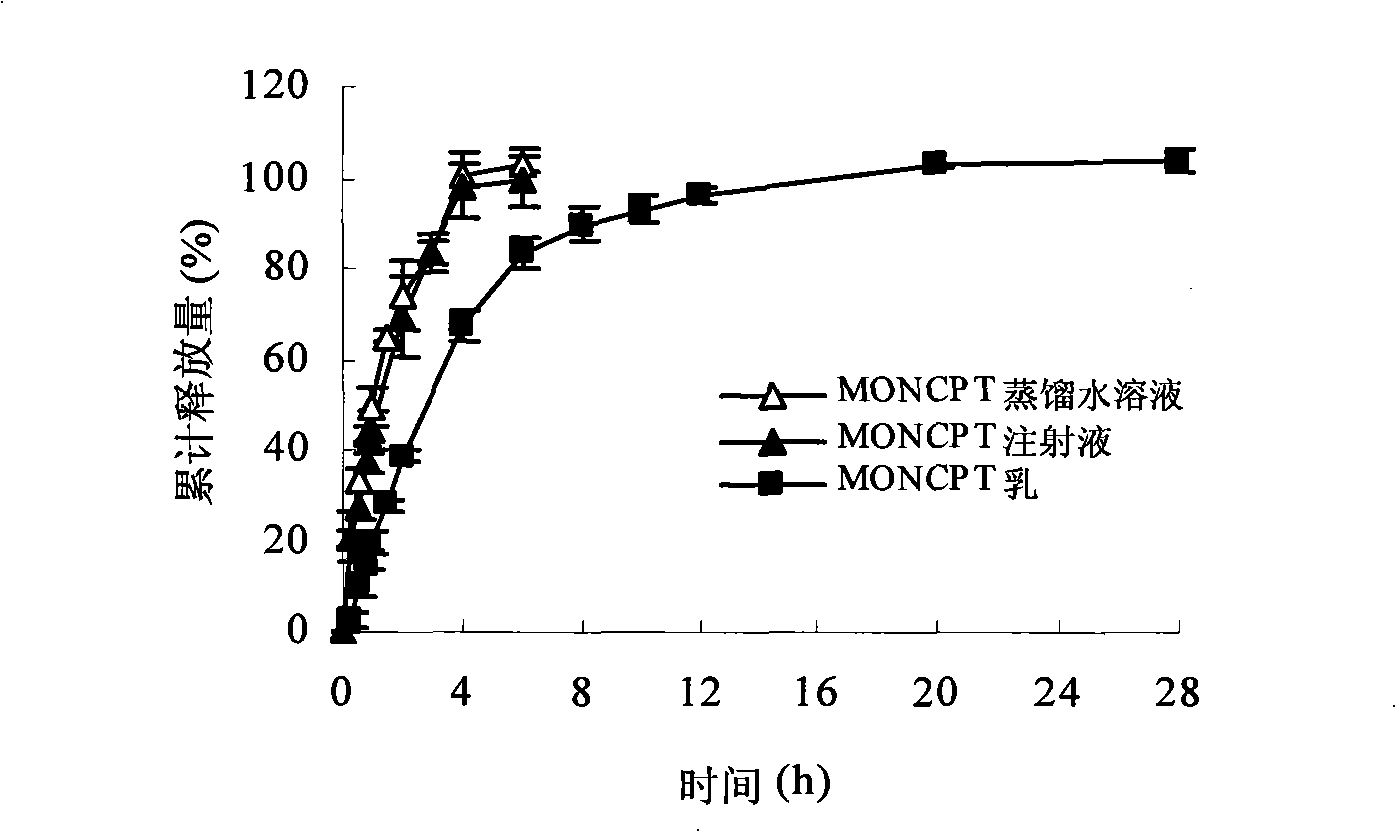
Camptothecinum derivative emulsion agent and preparation thereof
A derivative, camptothecin technology, applied in the field of new anticancer compound emulsion and its preparation, can solve the problems of reduced activity of the open-ring form, difficult to continue clinical research, severe bone marrow suppression, etc. release and improve the stability of the drug, the effect of high drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 0.27g of lecithin, 0.5g of Cremophor EL and 0.4g of ethanol in 1.6g of soybean oil, add 8.6g of polyethylene glycol 400, 9g of glycerin formal and 15mg of MONCPT, heat to 70°C to fully dissolve, then add 0.66 G glycerin and 12g water, high-speed homogeneous emulsification into colostrum, and further micro-emulsion made by micro-jet technology.
[0021] 1 Particle size: 80% of the particle size of the emulsion measured by a Malvern particle size analyzer is within the range of 50-85nm.
[0022] 2pH: The pH of the emulsion measured by a pH meter is 5.7.
[0023] Solubility determination of MONCPT in different solvents:
[0024] Take 10mg of MONCPT in a 10ml volumetric flask, dilute to the mark with different solvents, shake at a constant temperature of 25°C for 72h, take it out, centrifuge (10000rpm, 10min), take the supernatant, and analyze the drug content. See Table 1 for the results.
[0025] Table 1 Solubility of MONCPT in different solvents (unit: ug / mL)...
Embodiment 2
[0028] Take soybean lecithin 0.6g, Tween-80 0.6g, Proxamer 188 0.6g dissolved in 8g soybean oil and 4g medium chain oil, add 6mg MONCPT, heat to 70°C to fully dissolve, then add 3g glycerin and 45g Water, high-speed homogeneous emulsification into colostrum, and further made into microemulsion through micro-jet technology.
[0029] 1 Particle size: The particle size of the emulsion measured by a Malvern particle size analyzer is within the range of 146±17nm.
[0030] 2 pH: The pH of the emulsion measured by a pH meter is 5.6.
Embodiment 3
[0032] Dissolve lecithin 0.08g, Tween-80 0.12g, cholesterol 0.04g and 0.01g oleic acid in 4g soybean oil, heat to 70°C to fully dissolve, then add MONCPT 8mg to dissolve, and finally add water phase (glycerol 0.88g , distilled water 14g), after high-speed homogenization to obtain colostrum, the colostrum microjet is made emulsion.
[0033] 1 Particle size: The particle size of the emulsion measured by the Malvern particle size analyzer is within the range of 160±23nm.
[0034] 2 pH: The pH of the emulsion measured by a pH meter is 5.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com