Daidzein micelles and preparation method thereof
A technology of daidzein and micelles, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of poor solubility of daidzein, cloudy micellar suspension, and low drug loading rate, etc. problem, to achieve the effect of improving bioavailability, solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 (in this example, daidzein: phospholipids: the weight ratio of the additive is 1: 12: 10)
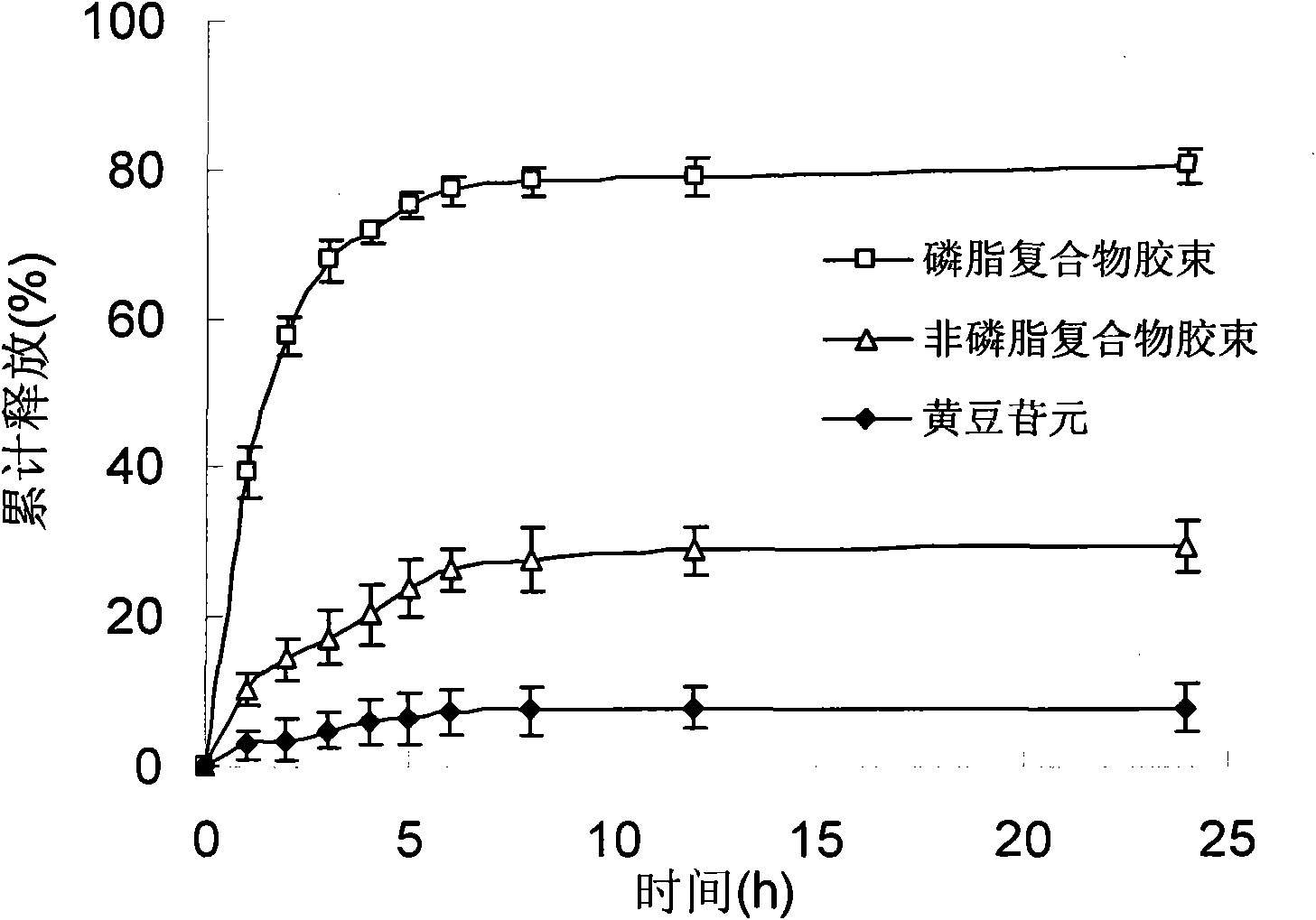
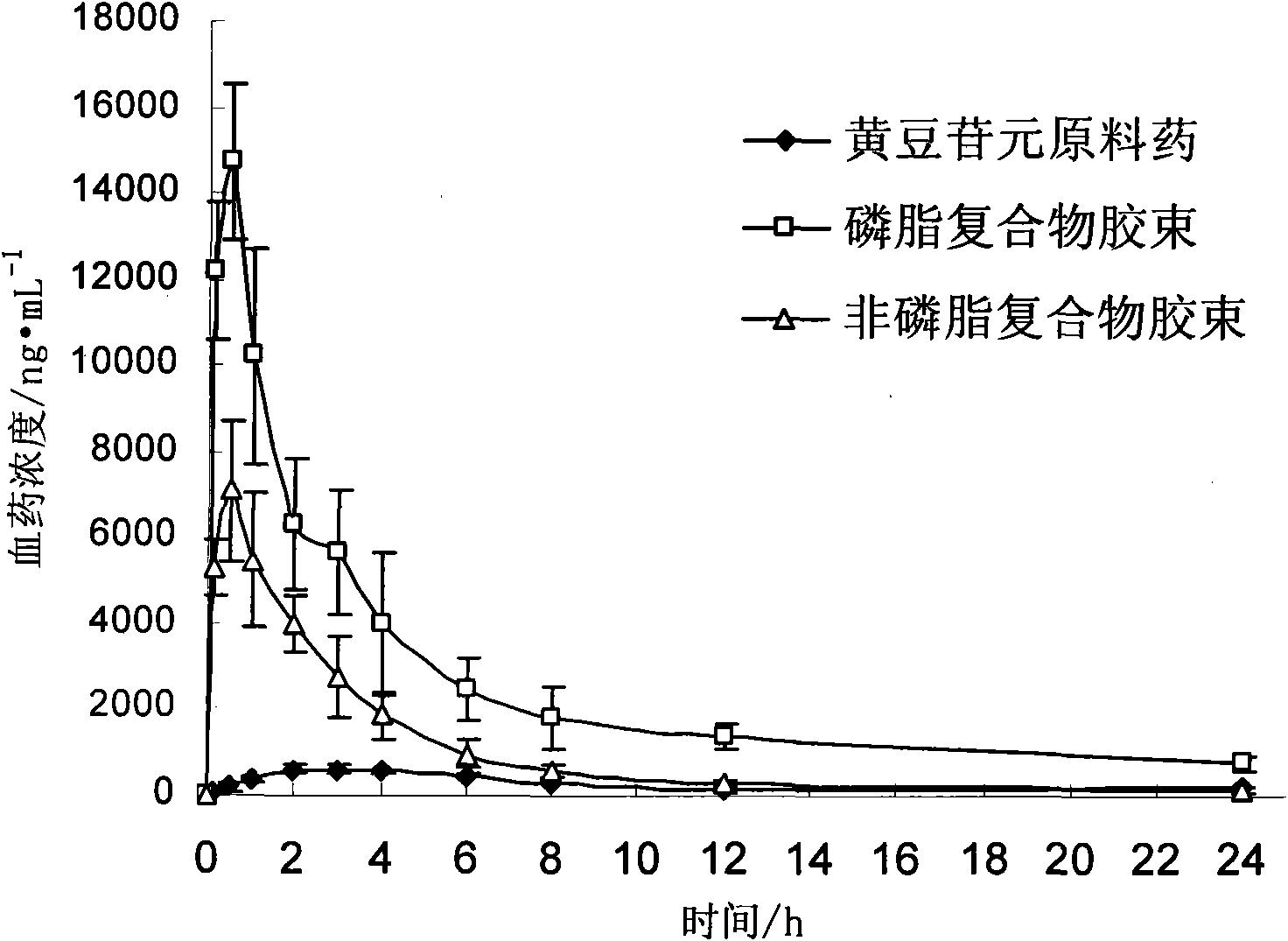
[0039] Take 1.0g of daidzein and 10g of soybean lecithin, add 10mL of acetone, heat to 60°C under reduced pressure and reflux, heat and stir for 10 hours, recover the acetone, dry, and pulverize to obtain the phospholipid complex; add soybean lecithin 2.0 to the phospholipid complex g, 10 g of glycerin, dissolved in 200 mL of ethanol, rotatively evaporated to form a film, and hydrated at 50 ° C to obtain daidzein micelles. The average particle diameter measured is 30nm, and the in vitro dissolution rate in 24 hours is 82%. Determination of its oral bioavailability, AUC of daidzein micelles 0-∞ 97152ng·h·mL -1 . Add water for injection and dilute to make 10mg / mL injection.
Embodiment 2
[0040] Embodiment 2 (in this embodiment, daidzein: phospholipid: the weight ratio of additive is 1: 25: 20)
[0041] Take 1.0g of daidzein and 20g of egg yolk lecithin, add 50mL of ethanol, heat to 40°C and reflux under reduced pressure, keep warm and stir for 5 hours, recover the ethanol, dry, and pulverize to obtain the phospholipid complex; add egg yolk egg to the phospholipid complex Dissolve 5.0 g of phospholipids and 20 g of sodium cholate in 200 mL of ethanol, rotatively evaporate to form a film, and hydrate at 50°C to obtain daidzein micelles. The average particle diameter measured is 20nm, and the in vitro dissolution rate in 24 hours is 92%. Determination of its oral bioavailability, AUC of daidzein micelles 0-∞ 131571ng·h·mL -1 . Add 0.02% sodium saccharin and distilled water to make 50mg / mL oral solution.
Embodiment 3
[0042] Example 3 (in this example, daidzein: phospholipids: the weight ratio of the additive is 1:30:4)
[0043] Take 1.0g of daidzein and 15g of soybean lecithin, add 20mL of methanol, heat to 60°C and reflux under reduced pressure, heat and stir for 2 hours, recover methanol, dry, and pulverize to obtain the phospholipid complex; add 15g of soybean lecithin to the phospholipid complex , Sodium cholate 4g, dissolved in 300mL ether, rotary evaporation to form a film, and hydrated at 50°C to obtain daidzein micelles. The measured average particle diameter is 30nm, and the in vitro dissolution rate in 24 hours is 84%. Determination of its oral bioavailability, AUC of daidzein micelles 0-∞ 116254ng·h·mL -1 . Add mannitol and water for injection, and freeze-dry to make freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com