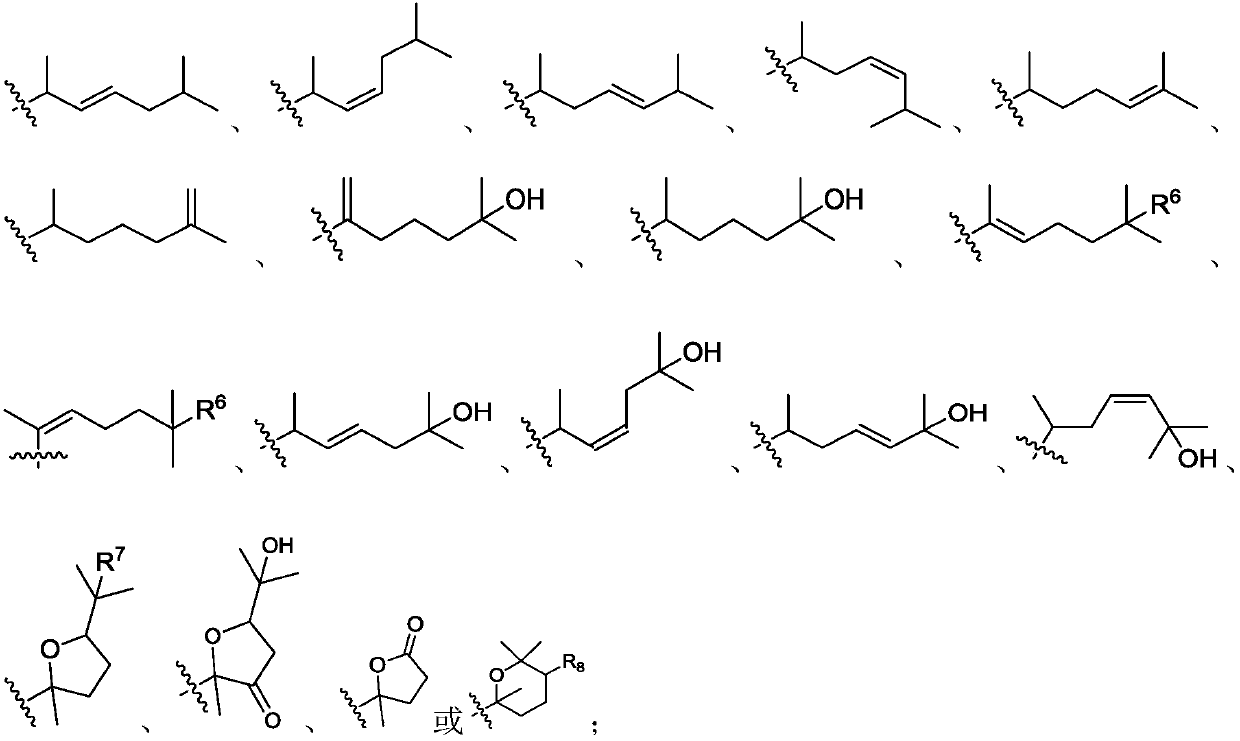
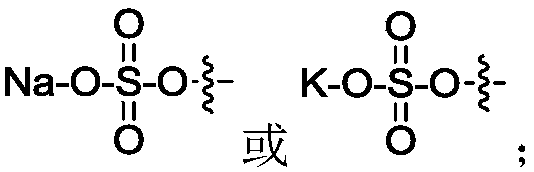
Novel blank liposomes taking ginsenoside derivatives as membrane materials, and preparation method and application thereof
A blank liposome, ginsenoside technology, applied in liposome delivery, skin care preparations, chemical instruments and methods, etc. higher sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0246] Preparation Example 1 Preparation of Isogeninsenoside Rg3(O)
[0247] Dissolve 10g of isoginsenoside Rg3 (type E) in 20mL of pyridine, add acetic anhydride dropwise under an ice-water bath, control the reaction temperature not to exceed 5°C, and react for 10 hours. TLC detects that the raw material point disappears, and then pour the reaction solution into ice water , extracted 3 times with 200 mL / times of ethyl acetate, combined the organic phases, washed 3 times with 200 mL / times of saturated NaCl, dried with an appropriate amount of anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain iso-Rg3 (type E) acetylated product.
[0248] Dissolve 5 g of the acetylated product of iso-Rg3 (type E) in 50 mL (THF: water 1:1), add 3 mL of 33% hydrogen peroxide, 2 mL of 2 M sodium hydroxide, and react overnight at room temperature. TLC detects that the raw material point disappears, Cool, wash 3 times with 100 mL / time of saturated sodium bicarbona...
preparation Embodiment 3
[0256] Preparation Example 3 Preparation of Ginsenoside Rp1
[0257] Take 1.5 g of ginsenoside Rg5, dissolve it in 150 mL of ethanol, add 300 mg of 5% palladium carbon, stir, and feed hydrogen gas at 40°C for 6 hours. After the reaction, the palladium carbon was removed by filtration, the filtrate was extracted three times with ethyl acetate, the organic phases were combined, and concentrated to dryness under reduced pressure to obtain crude ginsenoside Rp1. Separation by high-pressure chromatography, using C18 as a filler, gradient elution with methanol and water, detection by evaporative light scattering (ELSD), and concentration of the product section to dryness to obtain 0.36g+0.55g of ginsenoside Rp1 with an HPLC purity of more than 98%.
[0258] Ginsenoside Rp1,
[0259] 1 H NMR (δ, 500M): 5.38 (1H, d, J = 7.5Hz), 4.91 (1H, d, J = 7.5Hz), 4.55 (1H, m), 4.45-4.49 (2H, m), 4.23- 4.33(5H,m),4.12-4.14(2H,m),3.90-3.92(3H,m),3.27(1H,m),2.28-2.59(4H,m),2.17(2H,m),1.99- 2.02...
preparation Embodiment 4
[0262] Preparation Example 4 Preparation of Isoginsenoside Rg3H
[0263] Following the same method as Preparation Example 3, the acetylated product of isoginsenoside Rg3 was taken as the starting material, hydrogenated, post-treated, and separated by high-pressure chromatography to obtain the refined product of isoginsenoside Rg3H.
[0264] Isoginsenoside Rg3H,
[0265] 1 H NMR (δ, 500M): 5.37 (1H, d, J = 7.5Hz), 4.91 (1H, d, J = 7.5Hz), 4.55 (1H, m), 4.45-4.49 (2H, m), 4.23- 4.33(5H,m),4.12-4.14(2H,m),3.90-3.92(3H,m),3.27(1H,m),2.28-2.59(2H,m),2.17(1H,m),1.99- 2.02(3H,m),1.80-1.89(3H,m),1.78(3H,d,J=5.7),1.67-1.71(3H,m),1.64(3H,s),1.61(3H,s), 1.41(6H,s),1.35-1.50(10H,m),1.28(3H,s),1.20(1H,m),1.09(3H,m),1.02(1H,m),0.97(3H,s) ,0.96(3H,m),0.79(3H,s),0.74(1H,m).
[0266] 13 C NMR (δ, 125M): 106.3, 105.3, 89.2, 83.6, 82.3, 78.6, 78.4, 78.2, 78.0, 71.9, 71.2, 63.1, 63.0, 77.4, 56.6, 55.0, 51.9, 50.6, 49.4, 48.8, 40.2, 39.9, 39.4, 37.1, 35.4, 32.3, 31.6, 30.0, 29.8, 27.3, 26.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D10 | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
| D90 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com