Preparation method of polycationic liposome/calcium phosphate nanoparticle drug delivery vector
A technology of polycation and drug delivery carrier, applied in the field of gene drug carrier preparation, can solve the problems of reduced cell uptake, particle aggregation and precipitation, low transfection efficiency, etc., achieves broad application prospects, overcomes easy aggregation and precipitation, and improves stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
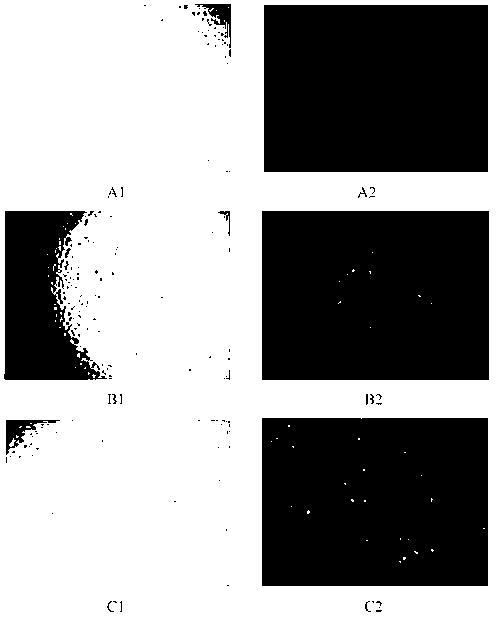
[0028] (1) Take 1ml of 75 mmol / L calcium chloride aqueous solution, add 0.1mg / ml oligonucleotide (ODN, 5'-CTCAGTTAGGGTTAG-3', 0.3nmol phosphate) solution 1ml, 0.4ml (4mg) 1% poloxamer 188, gently shaken and incubated for 30 minutes, as mixture A. Take another 2 ml of 6 mmol / L disodium hydrogen phosphate aqueous solution, add 0.5 ml of 25 mmol / L sodium citrate aqueous solution, and mix with 0.6 ml (6 mg) 1% poloxamer 188, as the mixed solution B. Add mixed solution B to mixed solution A dropwise, and stir at room temperature (25°C) for 30 min to obtain 5.5 ml of calcium phosphate nanoparticle solution (ODN mass: 0.1 mg). (2) Take soybean lecithin 10mg, cholesterol 5mg, polyethyleneimine-cholesterol (PEI-Chol) 1mg (PEI molecular weight 800 Da, amino molarity 20nmol) dissolved in 10ml chloroform, vacuum rotary evaporation to dry chloroform, add 10ml Deionized water was hydrated for 10 minutes under ultrasonic conditions in a water bath, and then further ultrasonic treatment was ...
Embodiment 2
[0031] (1) Take 1ml of 50 mmol / L calcium chloride aqueous solution, add 0.1mg / ml green fluorescent protein particle (pEGFP-N1, GenBank Accession #U55762, 4.7kb, molar mass of phosphate radical is 0.3nmol) solution 1ml, 0.4ml ( 4mg) 1% poloxamer 188, shake gently and incubate for 30 minutes as mixture A. Take another 2ml of 25mmol / L disodium hydrogen phosphate aqueous solution, add 2ml of 25mmol / L sodium citrate aqueous solution, and mix with 0.8ml (8mg) 1% poloxamer 188 as the mixed solution B. Add the mixed solution B to the mixed solution A dropwise, and stir at room temperature for 30 min to obtain a calcium phosphate nanoparticle solution (the mass of pEGFP-N1 plasmid is 0.1 mg). (2) Take polyethyleneimine-cholesterol 5mg (PEI molecular weight 800 Da, amino molarity 100nmol), DOPE 2.5mg dissolved in 10ml chloroform, vacuum rotary evaporation to dry the chloroform, add 5ml deionized water, in water bath ultrasonic condition Hydrate for 10 minutes, and then use an ultrasoni...
Embodiment 3
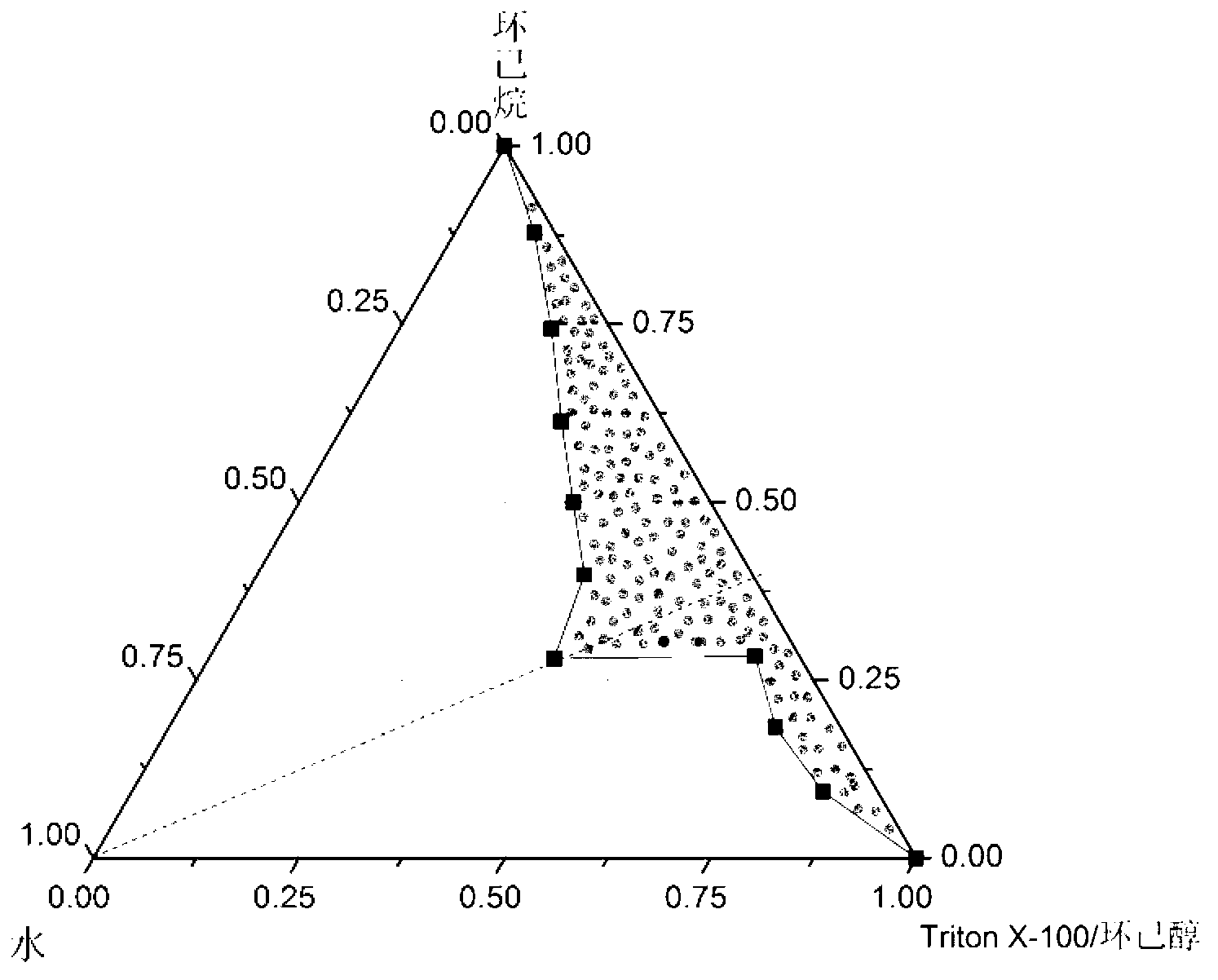
[0034] (1) Mix 2.52ml of polyethylene glycol octylphenyl ether (Triton X-1), 1.68ml of n-hexanol, and 2.8ml of cyclohexane to form 7ml of an oil phase ternary system. Mix 100 μL of 250 mmol / L calcium chloride aqueous solution, 100 μL of 25 mmol / L disodium hydrogen phosphate aqueous solution, 500 μL of 0.1 mg / ml pEGFP-N1 plasmid (the molar mass of phosphate radical is 0.15 nmol) and 100 μL of 15 mmol / L sodium citrate aqueous solution Mix to make a mixture. Add the mixed solution drop by drop into the ternary system under stirring, stir at room temperature for 0.5 hours, dialyze the mixed solution with a dialysis bag (molecular weight cut-off 14000 Da) for 72 hours to remove the organic phase, the dialysate is absolute ethanol, take the retained solution and Disperse in 5ml of deionized water to obtain the calcium phosphate nanoparticle solution (the mass of pEGFP-N1 plasmid is 0.05mg). (2) Take PEI-Chol 5mg (PEI molecular weight 800 Da, amino molarity 100nmol), DOPE 2.5mg diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com