Biological adhesive liposome preparation for eyes and preparation method thereof
A liposome preparation and bioadhesion technology, which is applied in the directions of liposome delivery, medical preparations of inactive ingredients, and drug combinations, etc., can solve the problem of poor compliance of semi-solid dosage forms, low ocular bioavailability, and difficulty in Patient acceptance and other issues to achieve the effect of improving ocular bioavailability, improving bioavailability, and promoting ocular absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
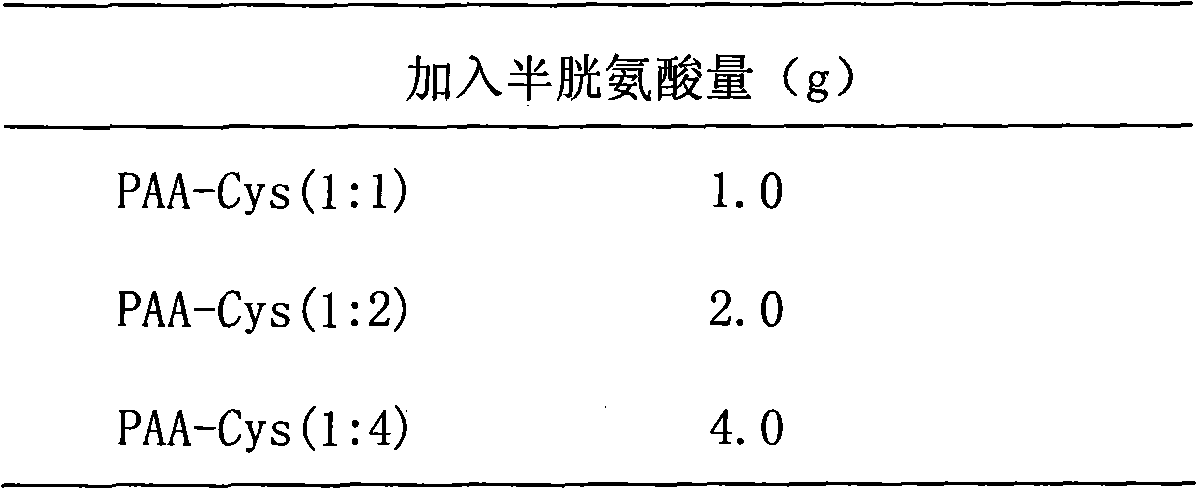
[0026] Preparation, separation and purification of cysteine-polyacrylic acid (Cys-PAA) complex: Take three parts of 1g PAA and add it to 100ml water, adjust the pH value to 7.2-7.5 with 2M NaOH, and disperse it in water. 3.8 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC) was added each to make the concentration 200 mM, and stirred at room temperature for 45 minutes. Use 5M HCl to adjust the pH value to 4-5, fill with nitrogen for 15 minutes, then add Cys, fill with nitrogen and stir at room temperature for 3 hours. The amount of cysteine added to each of the three parts is shown in Table 1.
[0027] Table 1
[0028]
[0029] The product was isolated by dialysis. The reaction solution was transferred to a dialysis bag, and dialyzed in 2000 ml of dialysis fluid at 4° C. in the dark. The first dialysate was 1 mM HCl containing 2 μM EDTA. The second dialysate was 1 mM HCl, containing 2 μM EDTA, and containing 1% NaCl, and the same medium was dialy...
Embodiment 2
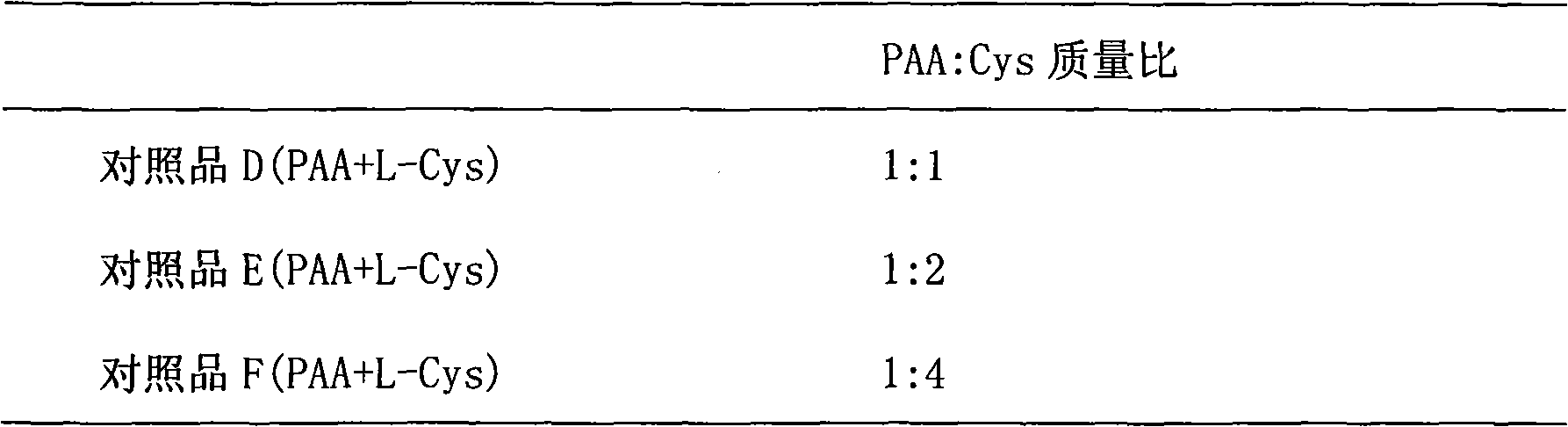
[0035]Determination of sulfhydryl content in Cys-PAA complex: The amount of sulfhydryl can be determined by DTNB method. Take 2mg each of the product and the reference substance and disperse them in 10ml of 0.5M phosphate buffer (PH8.0), respectively, take 0.5ml each, add 0.5ml of 0.03% 5,5-dimercapto-2,2-dinitrobenzoic acid (DTNB) solution, put it at room temperature for 2 hours, take 0.3ml and transfer it into a 96-well plate, put it into a microplate reader to measure the absorbance (absorbance value is set at 405nm). The cysteine solution standard curve Y=163.38X+28.456 was obtained, and the sulfhydryl content in Cys-PAA was calculated based on this. The thiol content obtained after deducting the absorbance of the blank reference substance (D, E, F) is shown in Table 3:
[0036] table 3
[0037]
Embodiment 3
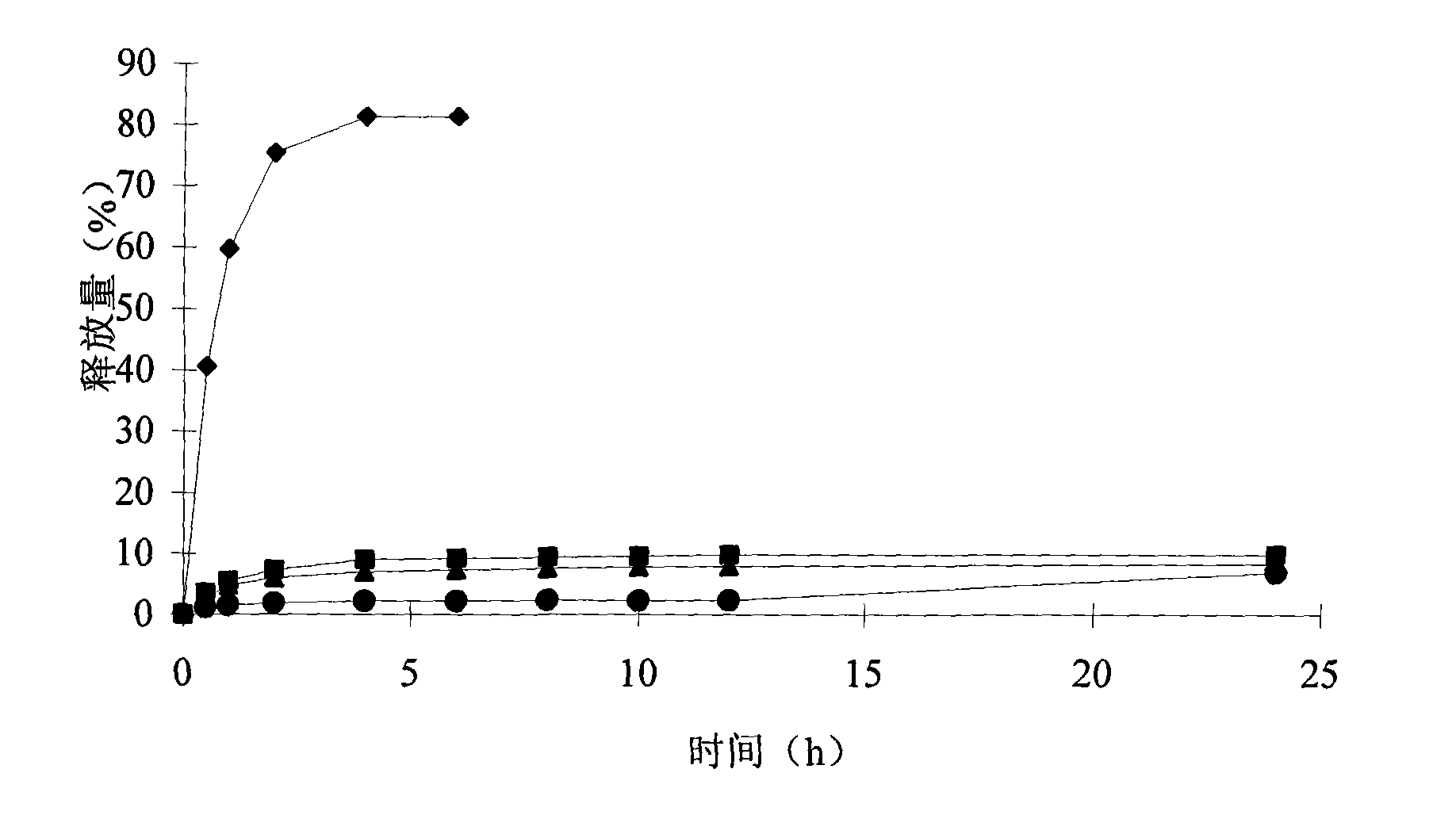
[0039] Evaluation of bioadhesion of PAA-Cys complex: Adult rats were taken, sacrificed after anesthesia, dissected, and the small intestinal mucosa was collected and stored at -20°C until use.
[0040] Take appropriate amounts of PAA-Cys (1:1), PAA-Cys (1:2), PAA-Cys (1:4), and PAA respectively to make a solution containing 0.2% PAA. Then it was divided into two parts, one was adjusted to neutral pH with 2M NaOH, and the other was adjusted to pH 3 with 5M HCl. Also adjust the pH value of pure water to neutral and 3. Take 0.2ml of each of the prepared above-mentioned solutions and drop them on the surface of the small intestinal mucosa, measure the adhesion force (mg) at 10 minutes and 30 minutes with a torsion balance, and repeat the measurement 6 times for each sample. The obtained adhesion results are shown in Table 4:
[0041] Table 4 (Neutral)
[0042]
[0043] (pH3)
[0044]
[0045] (Net Adhesion (mg))
[0046]
[0047]
[0048] The results showed that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com