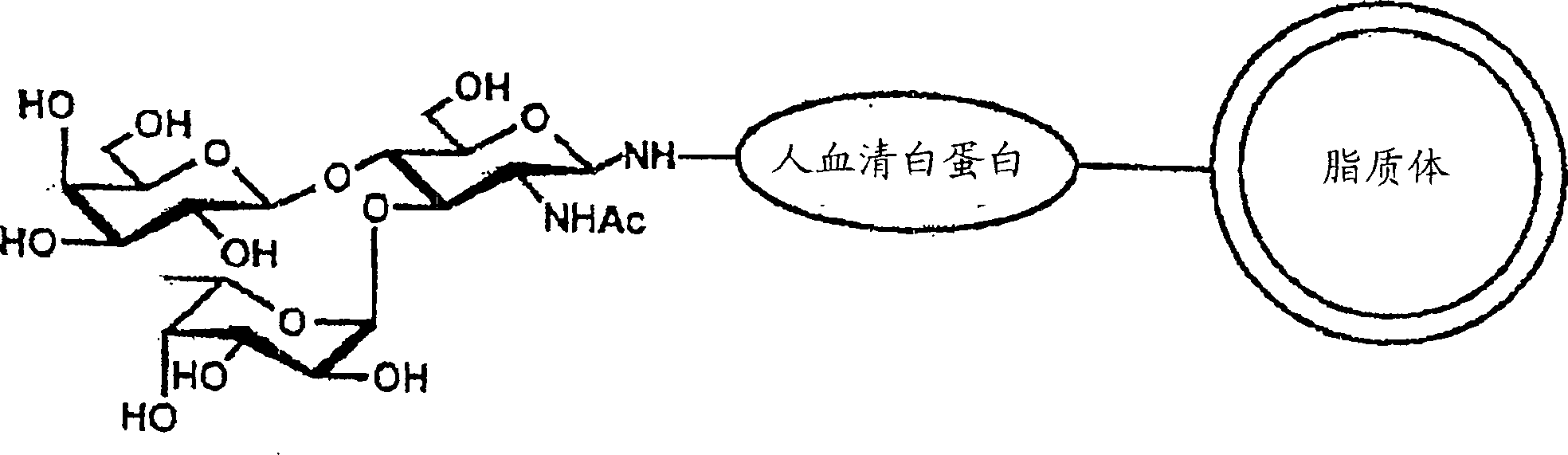
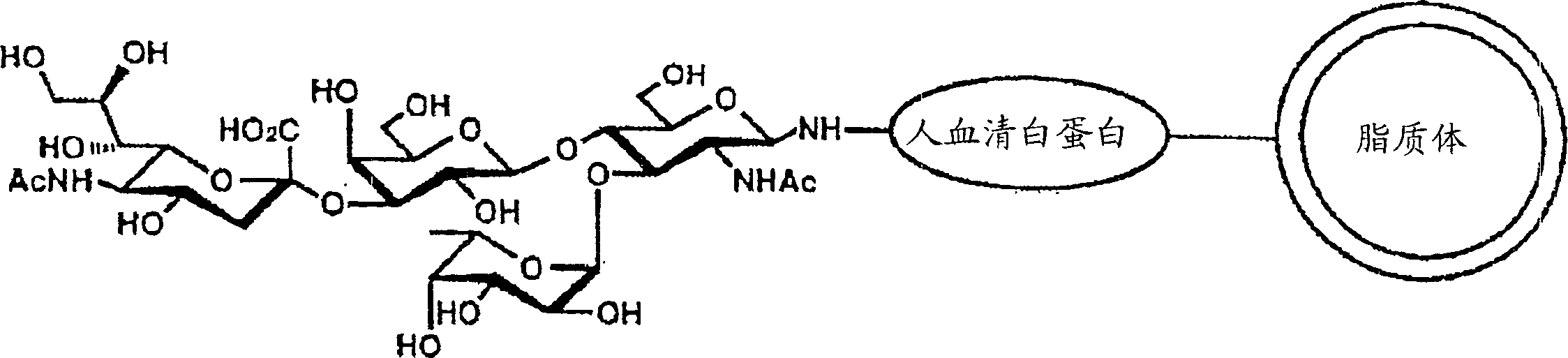
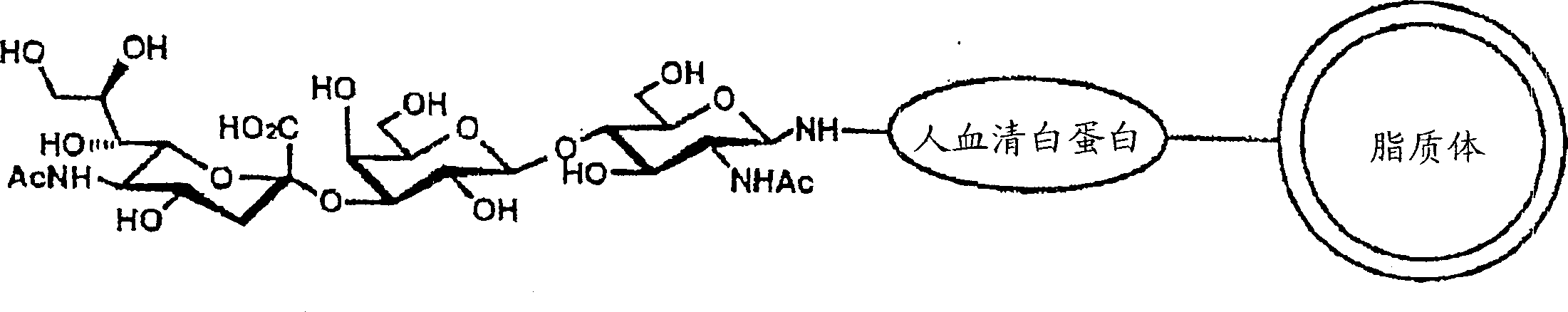
Remedy or diagnostic for inflammatory disease containing target-directing liposome
An inflammatory disease, liposome technology, applied in the field of drug delivery systems for inflammatory diseases, can solve problems such as unclear pathogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] The preparation of embodiment 1 liposome
[0184] Liposomes were prepared using a modified cholic acid dialysis method according to a reported method (Yamazaki, N., Kodama, M. and Gabius, H.-J. (1994) Methods Enzymol. 242, 56-65). That is, dipalmitoylphosphatidylcholine, cholesterol, dihexadecyl phosphate, ganglioside and diacetate with a molar ratio of 35:40:5:15:5 and a total lipid content of 45.6 mg Add 46.9mg of sodium cholate to palmitoylphosphatidylethanolamine and dissolve in 3ml of chloroform / methanol solution. The solution was evaporated and the precipitate was dried in vacuo to obtain a lipid film. The obtained lipid film was suspended in 3 ml of TAPS buffer (pH 8.4), and subjected to ultrasonic treatment to obtain a transparent micellar suspension. The micellar suspension was subjected to ultrafiltration using PM10 membrane (Amicon Co., USA) and PBS buffer (pH 7.2) to prepare 10 ml of uniform liposomes (average particle size 100 nm).
Embodiment 2
[0185] The hydrophilicity treatment on embodiment 2 liposome lipid membrane face
[0186] Using XM300 membrane (AmiconCo., USA) and CBS buffer (pH8.5), 10 ml of the liposome solution prepared in Example 1 was subjected to ultrafiltration so that the pH of the solution was 8.5. Next, 10 ml of cross-linking reagent bis(sulfosuccinimidyl) suberate (BS3; Pierce Co., USA) was added and stirred at 25° C. for 2 hours. Then, stir overnight at 7° C. to terminate the chemical bonding reaction between lipid dipalmitoylphosphatidylethanolamine and BS3 on the liposome membrane. The liposome fluid was ultrafiltered with XM300 membrane and CBS buffer (pH 8.5). Next, 40 mg of tris(hydroxymethyl)aminomethane dissolved in 1 ml of CBS buffer (pH8.5) was added to 10 ml of liposome liquid, stirred at 25° C. for 2 hours, and then stirred overnight at 7° C. The chemical bonding reaction of lipid-bound BS3 and tris(hydroxymethyl)aminomethane on the liposome membrane is terminated. As a result, the...
Embodiment 3
[0187] The combination of embodiment 3 human serum albumin (HSA) and liposome membrane face
[0188] The coupling reaction was carried out according to the reported method (Yamazaki, N., Kodama, M. and Gabius, H.-J. (1994) Methods Enzymol. 242, 56-65). That is, this reaction is carried out with 2 step chemical reactions, at first, the sodium metaperiodate that 43mg is dissolved in the 1ml TAPS damping fluid (pH8.4) is added in the 10ml liposome that embodiment 2 obtains, stir at room temperature For 2 hours, gangliosides present on the membrane surface were subjected to periodic acid oxidation, and then ultrafiltered through an XM300 membrane and PBS buffer (pH 8.0) to obtain 10 ml of oxidized liposomes. Add 20 mg of human serum albumin (HSA) to the liposome liquid, stir at 25°C for 2 hours, then add 100 μl of 2M NaBH to PBS (pH 8.0) 3 CN, stirred overnight at 10°C, binds HSA through the coupling reaction of gangliosides on liposomes to HSA. Ultrafiltration was carried out w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Zeta potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com