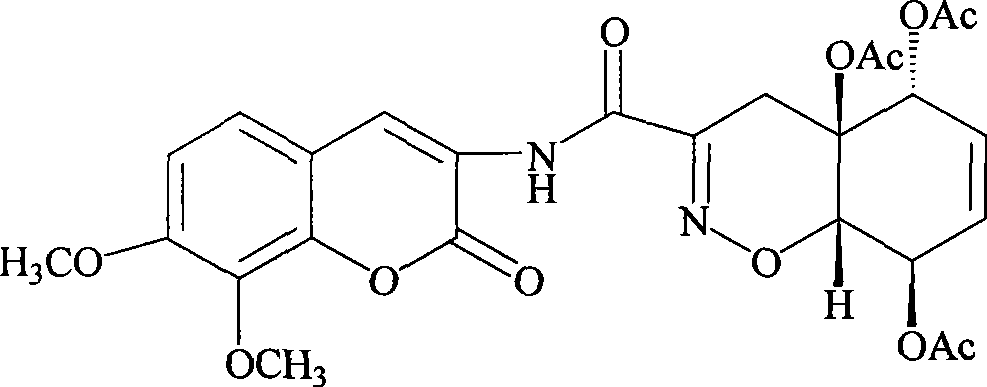
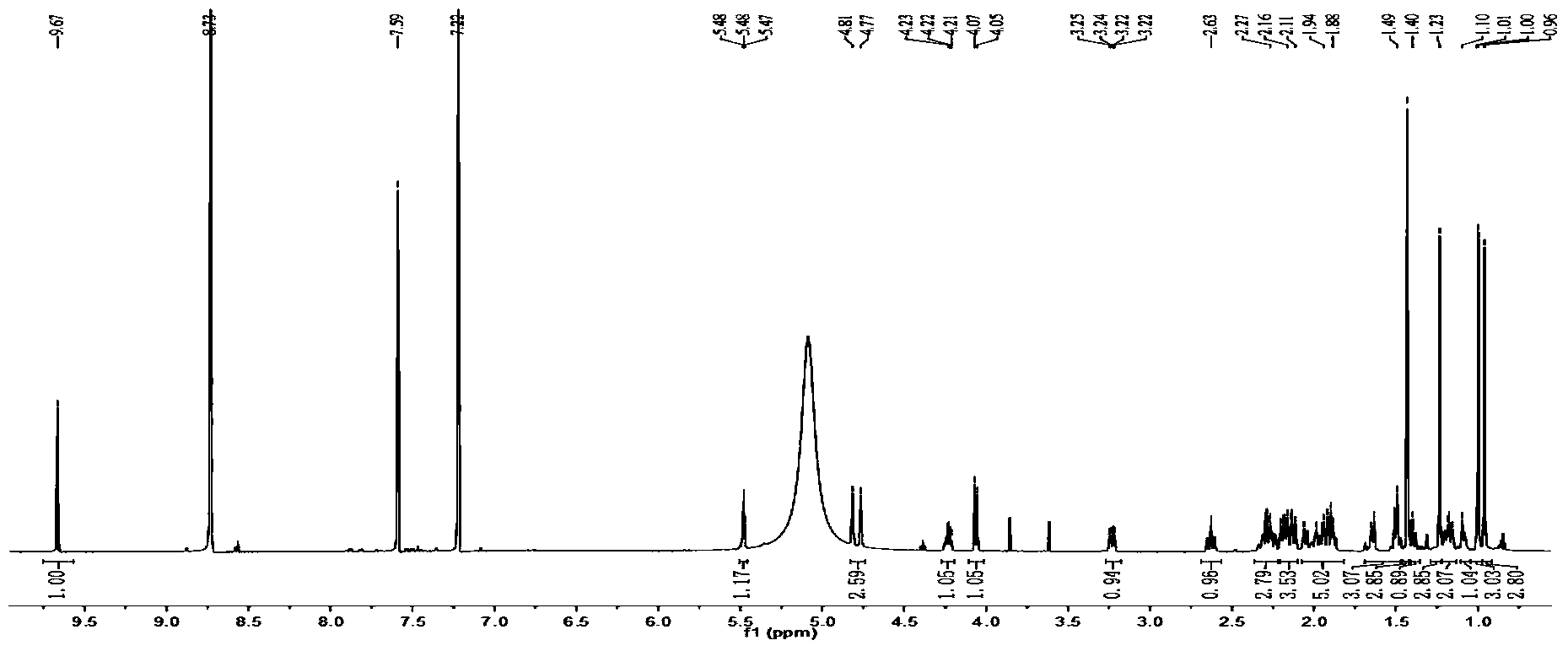
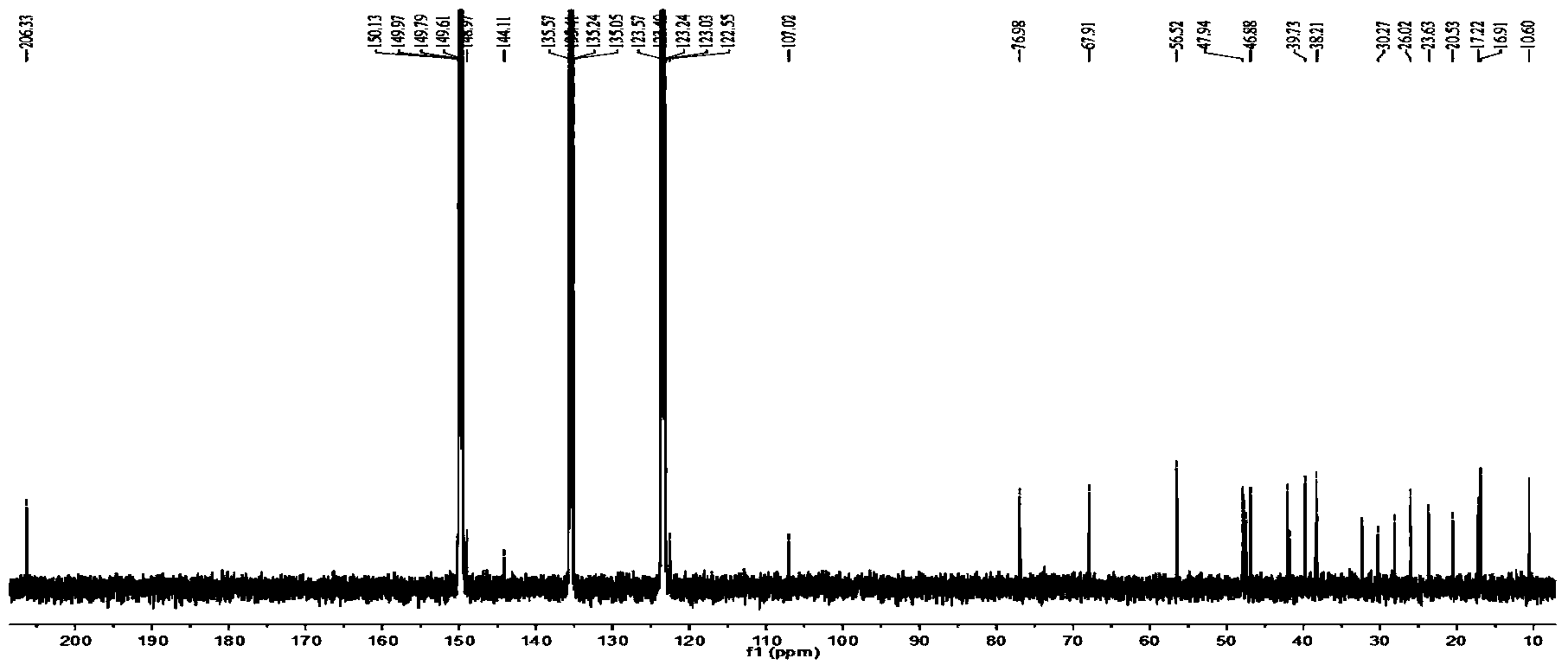
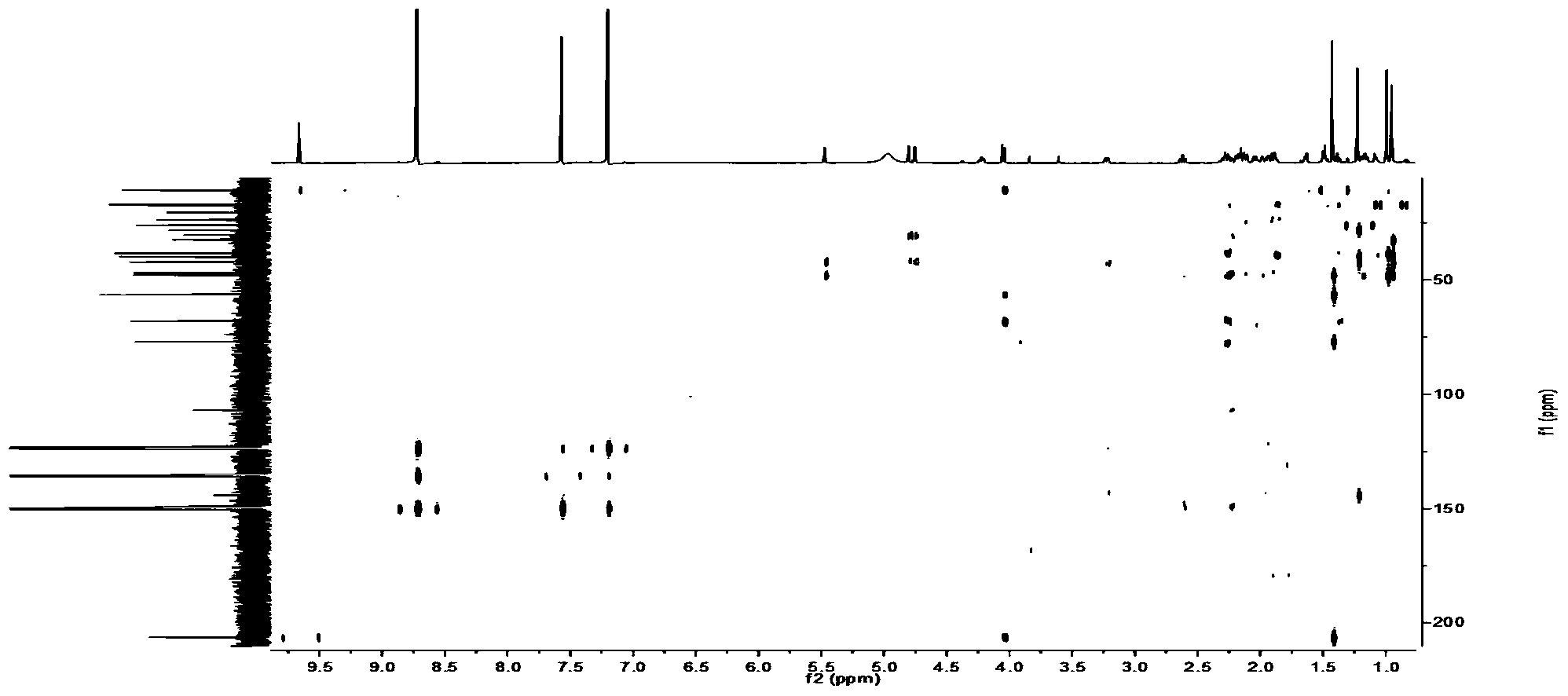
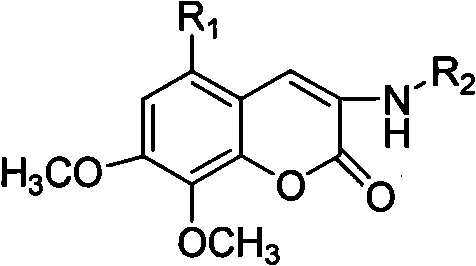
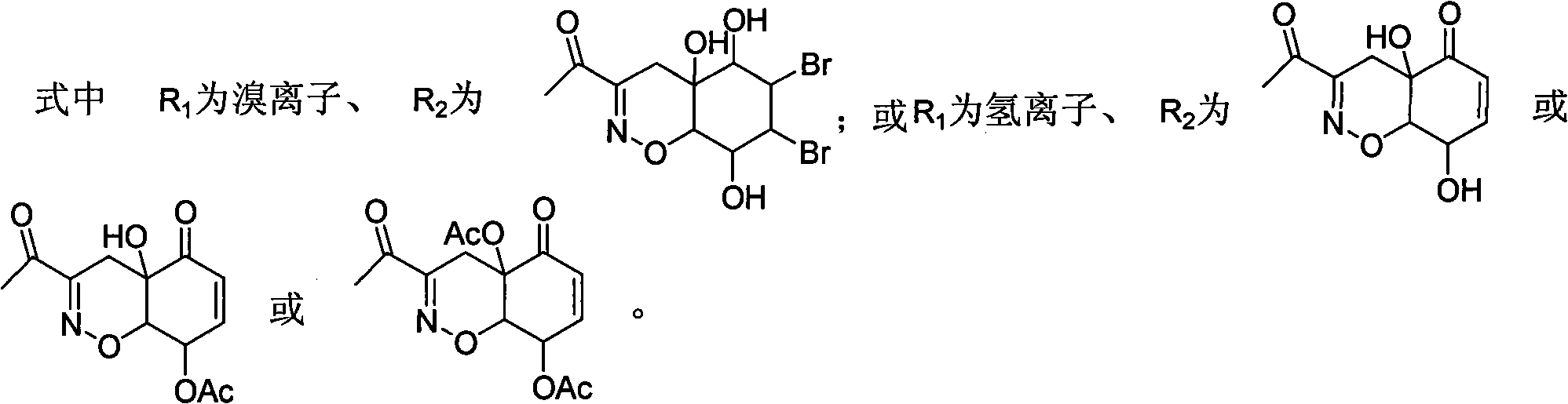
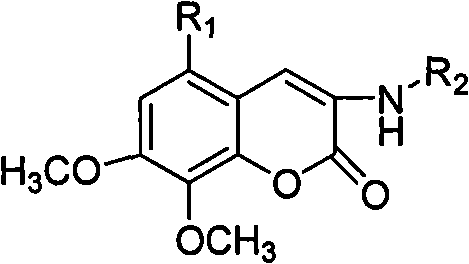
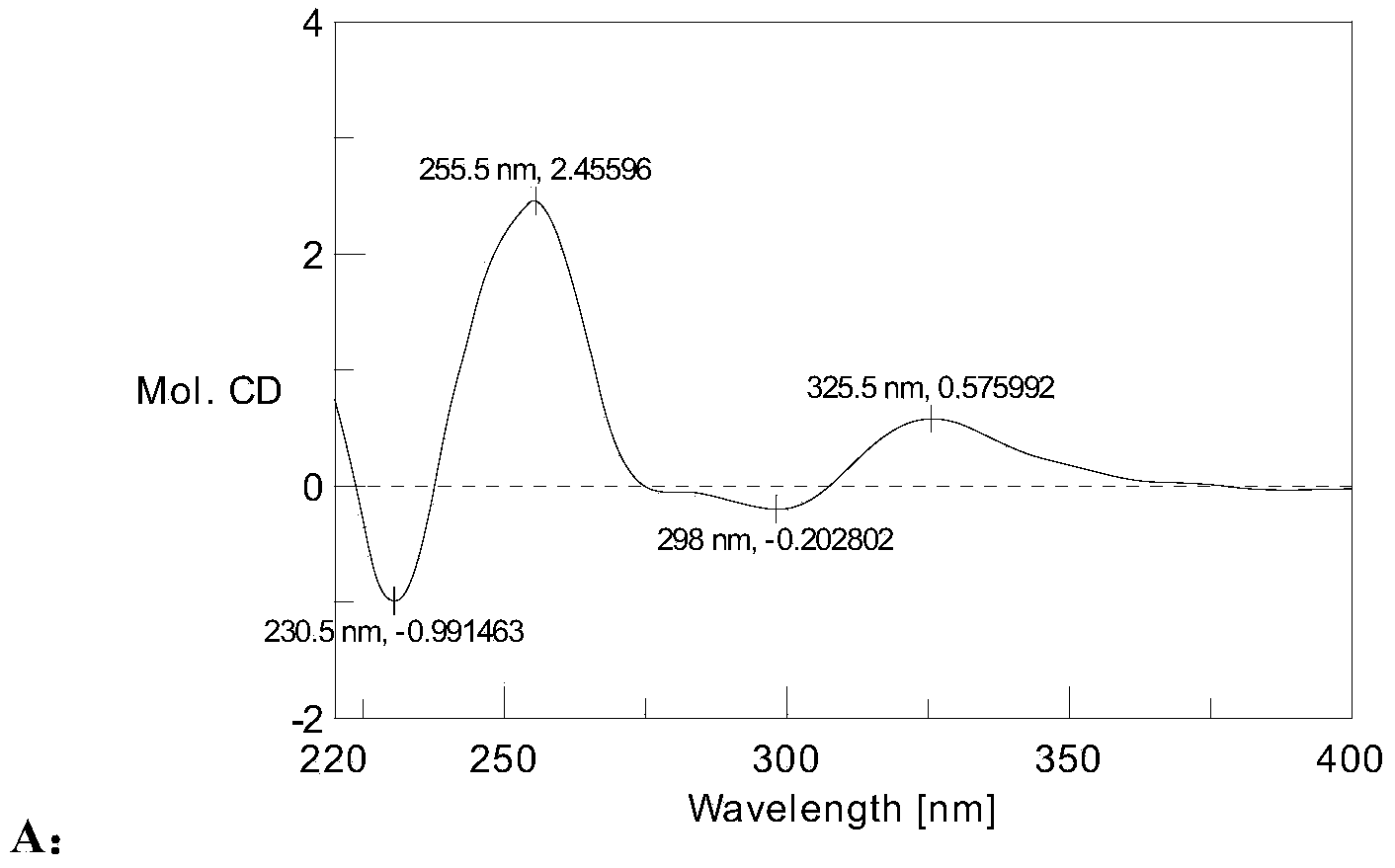
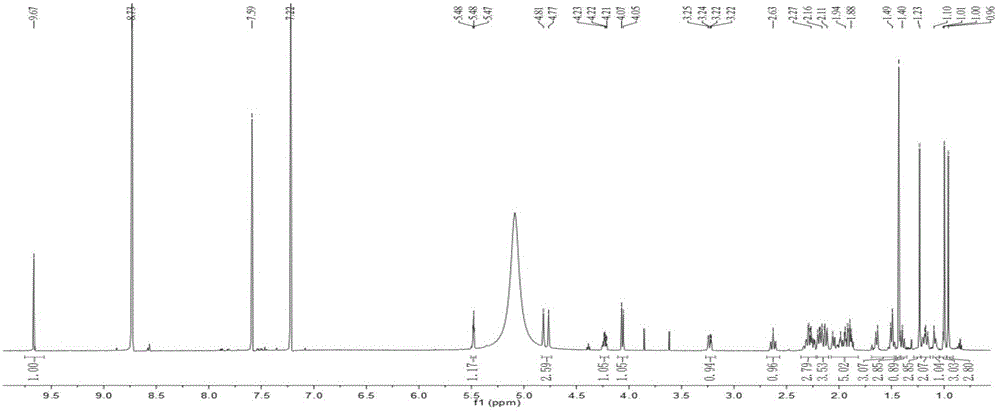
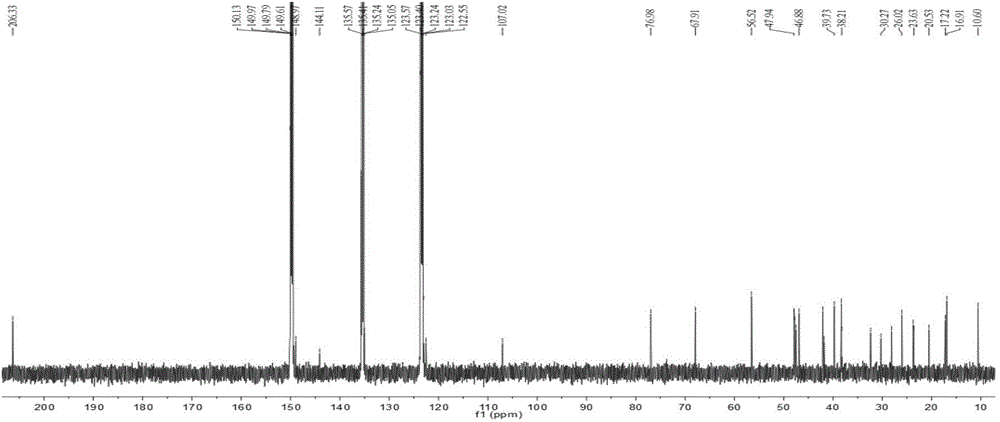
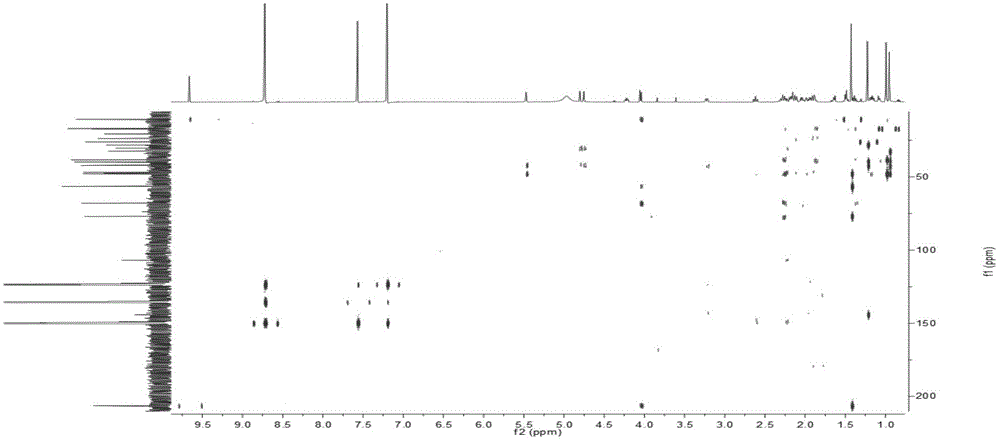
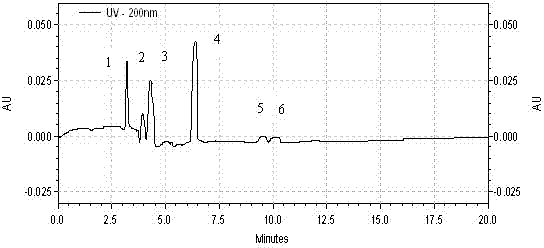
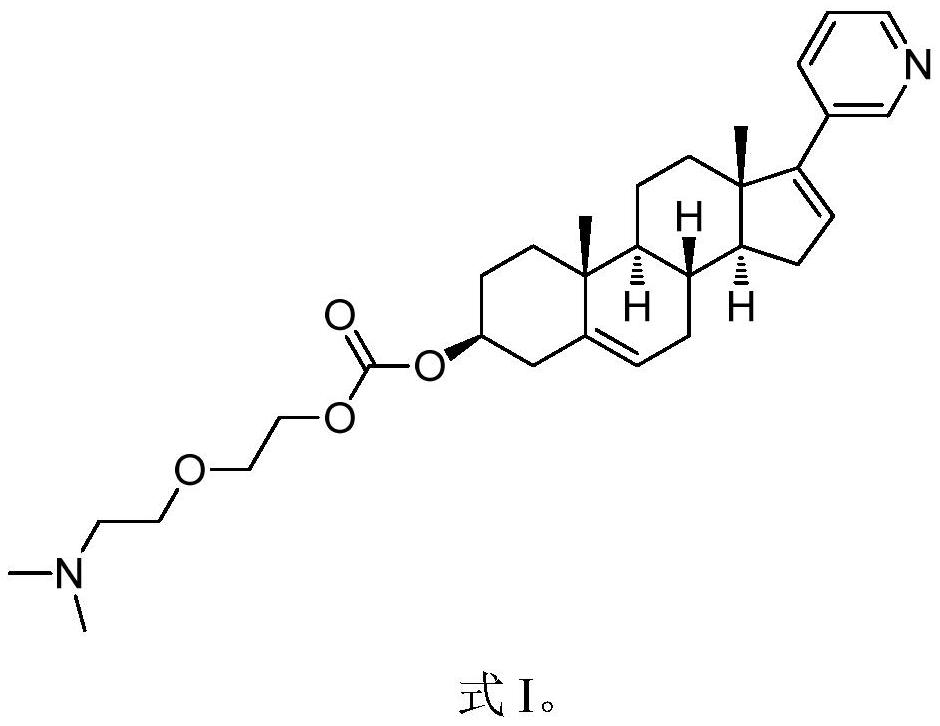
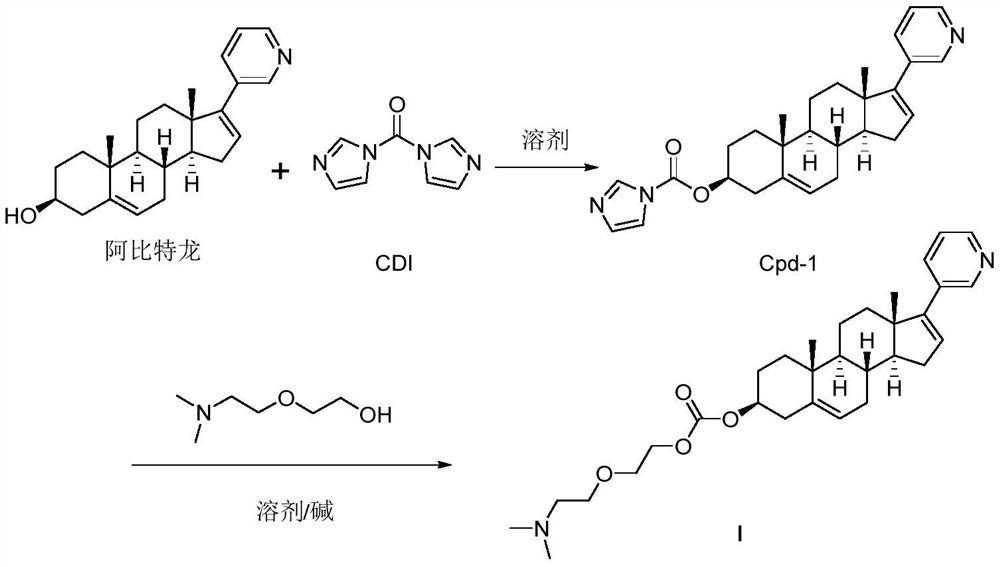
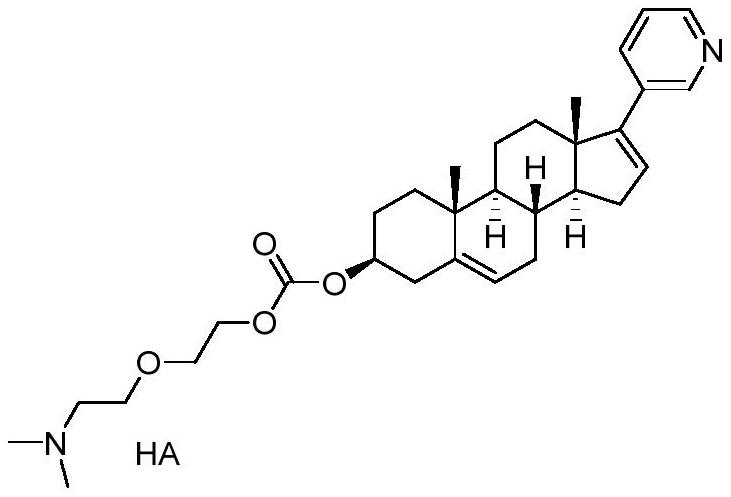
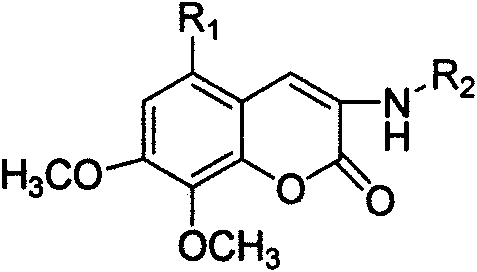
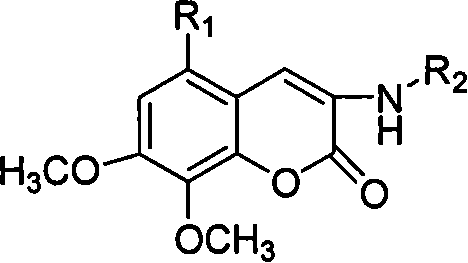
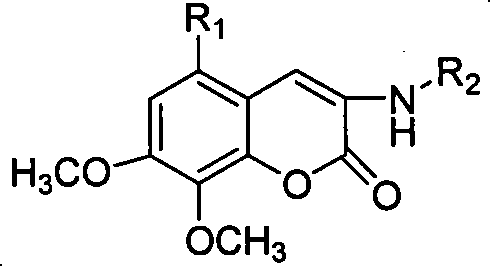
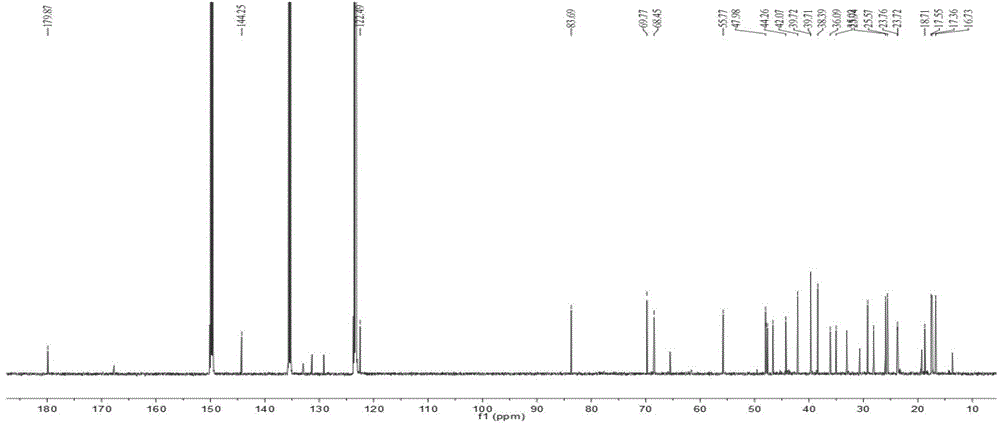
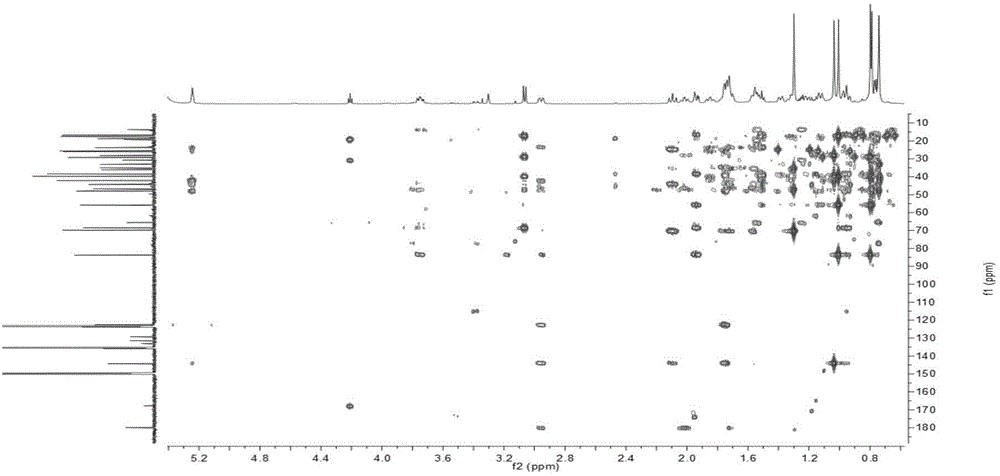
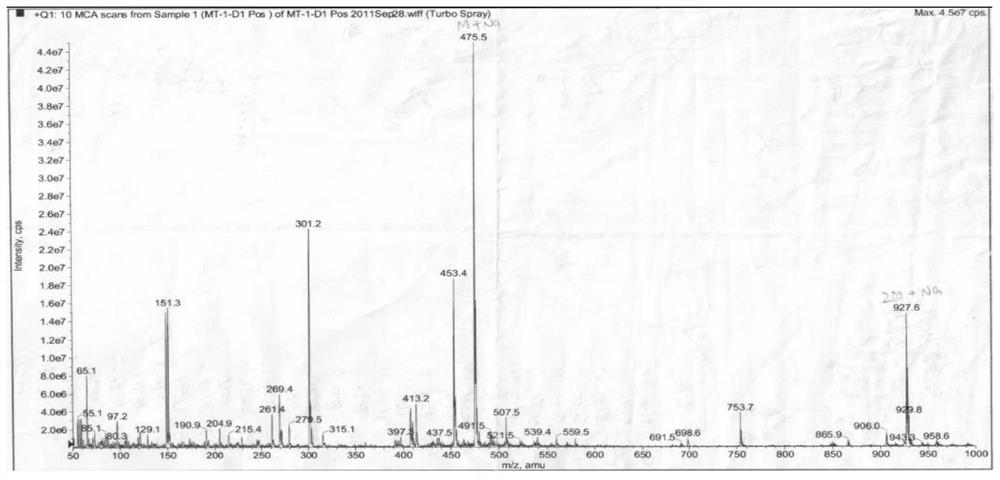
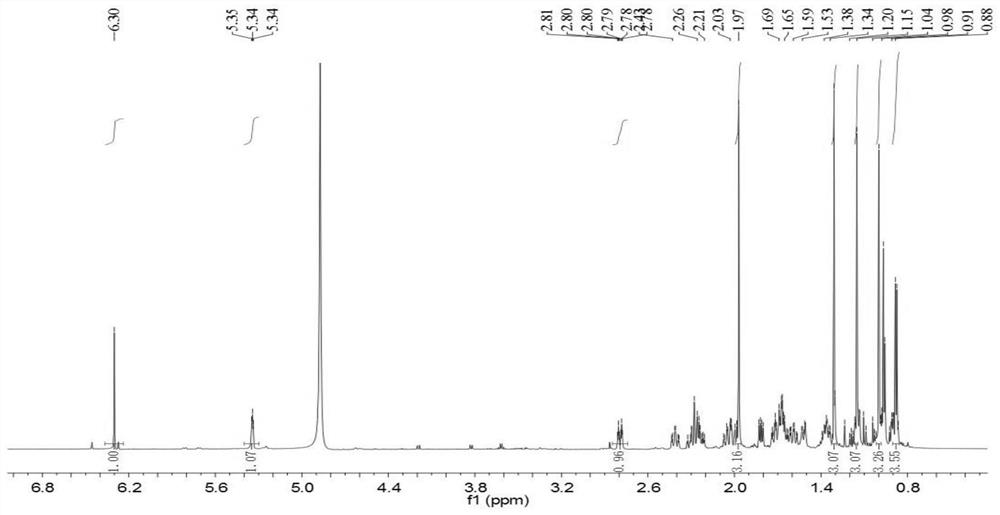
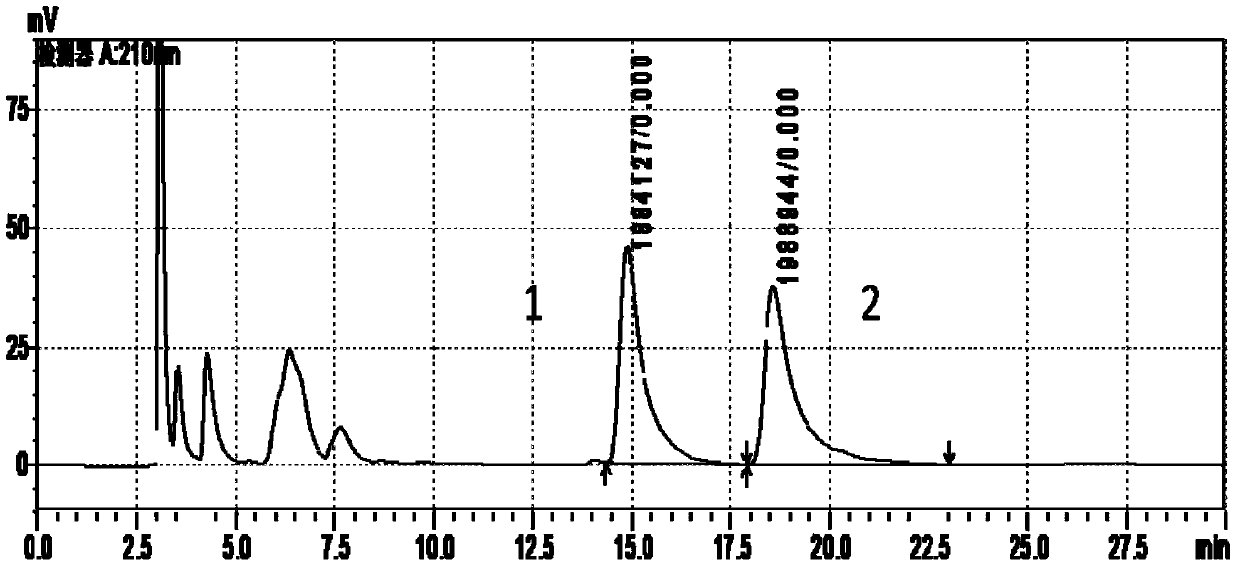
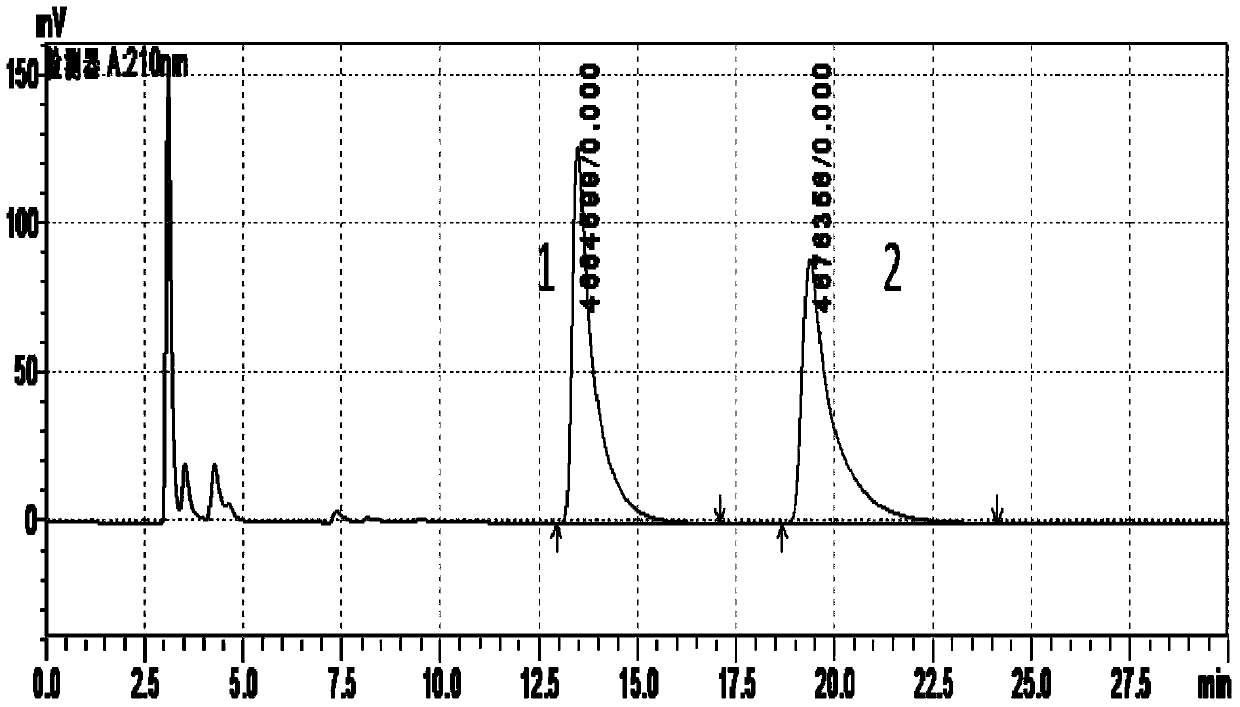
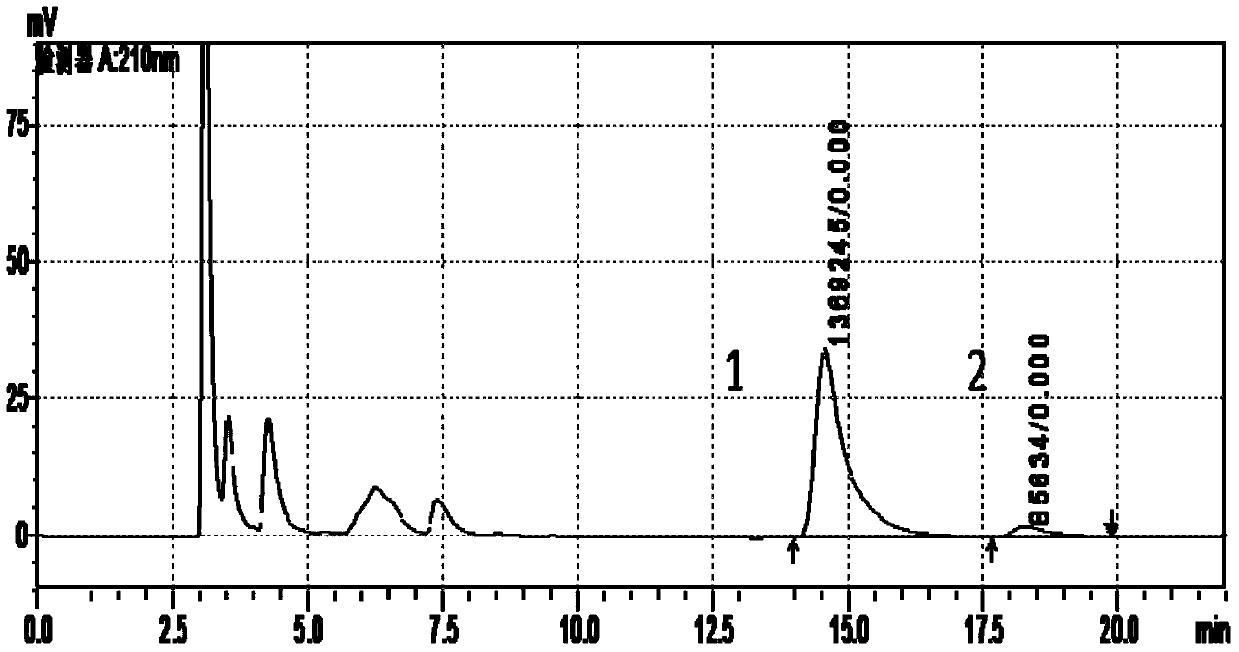
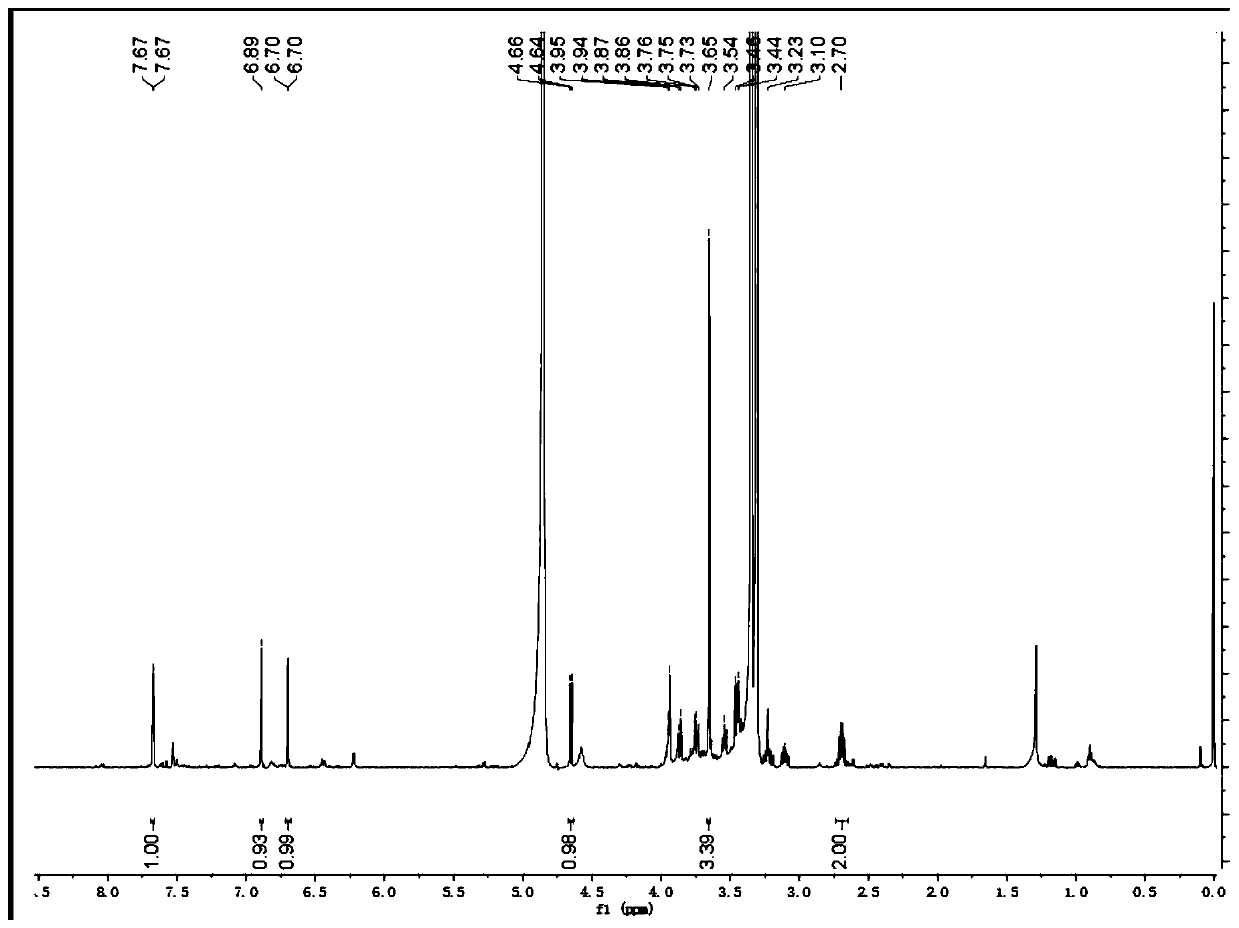
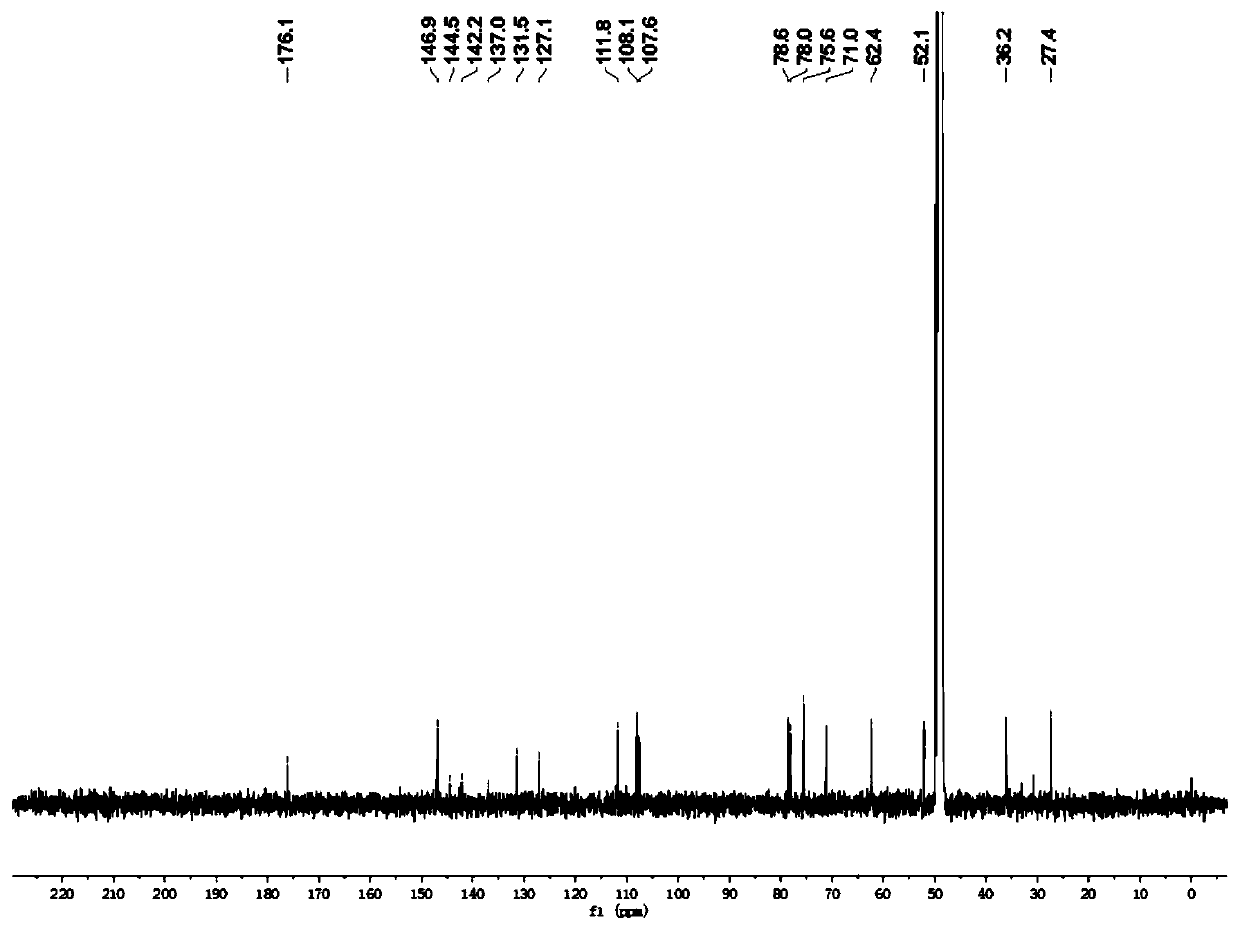
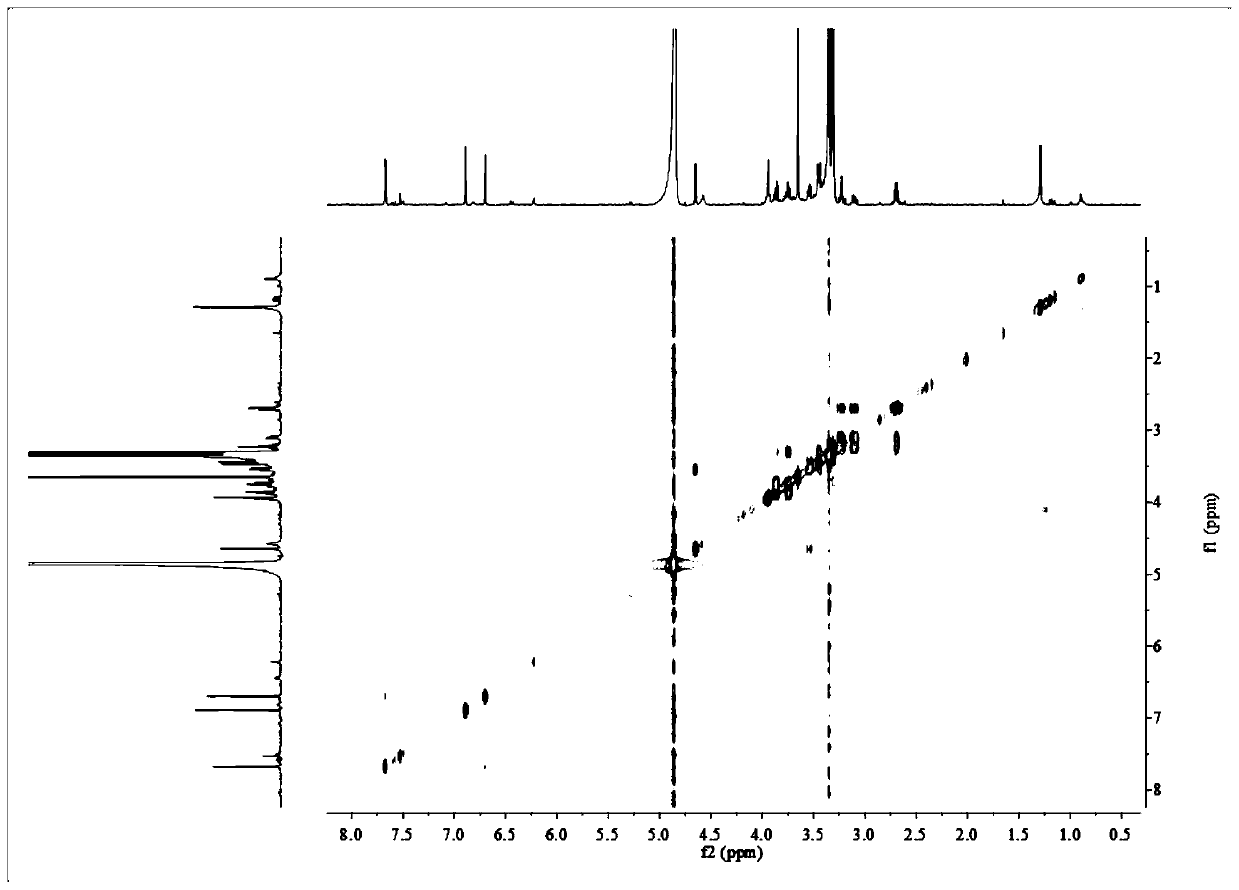
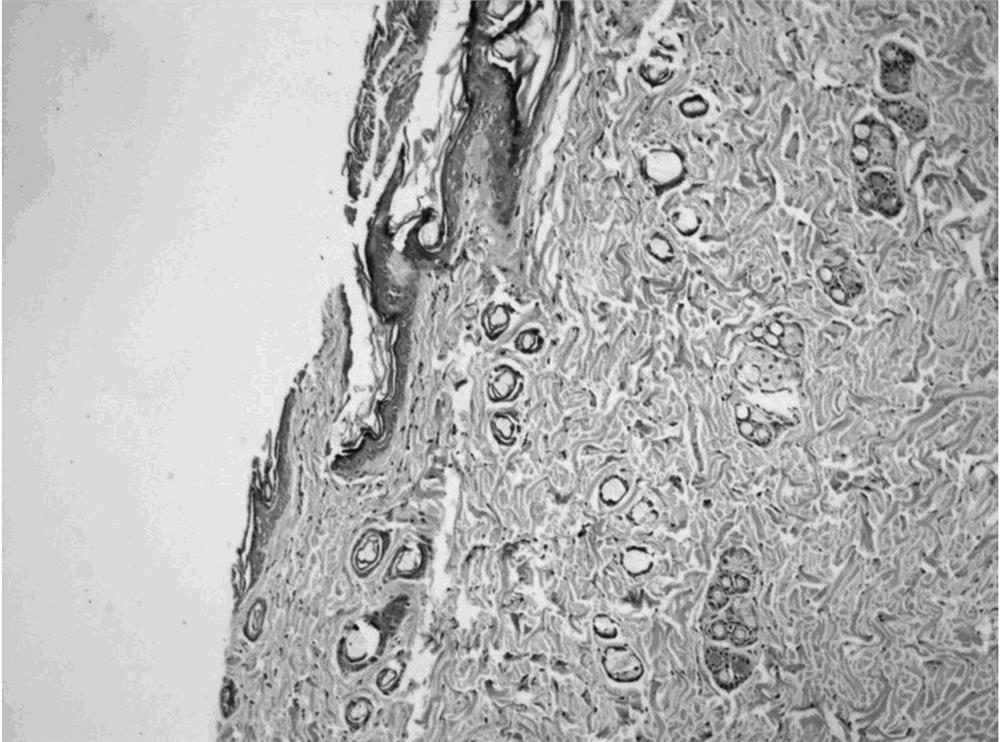
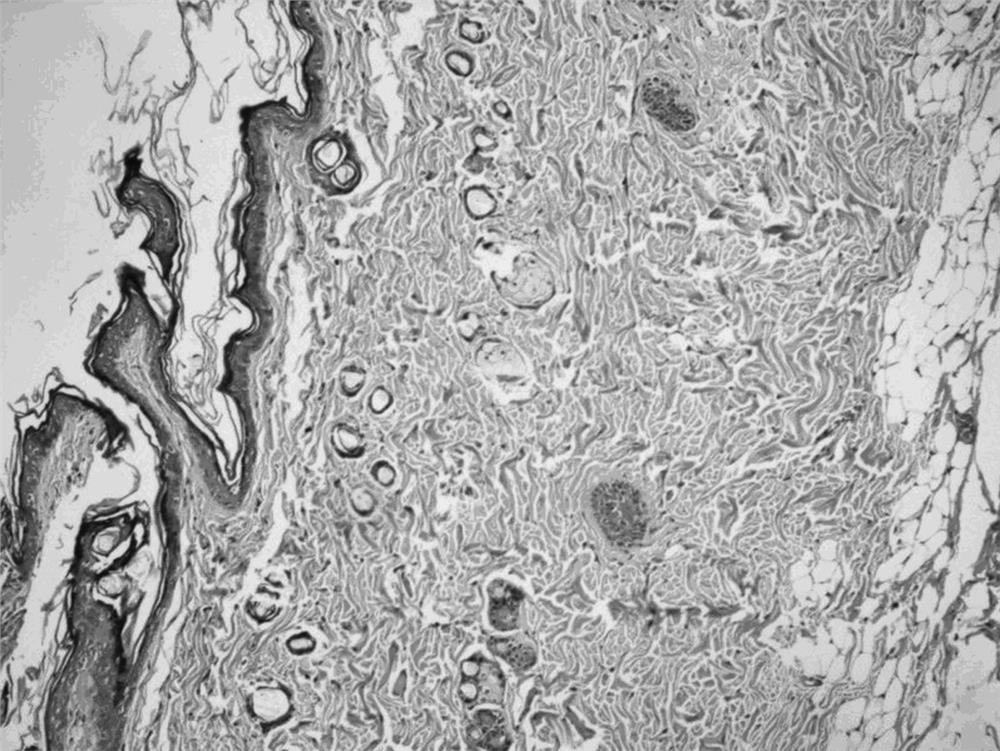
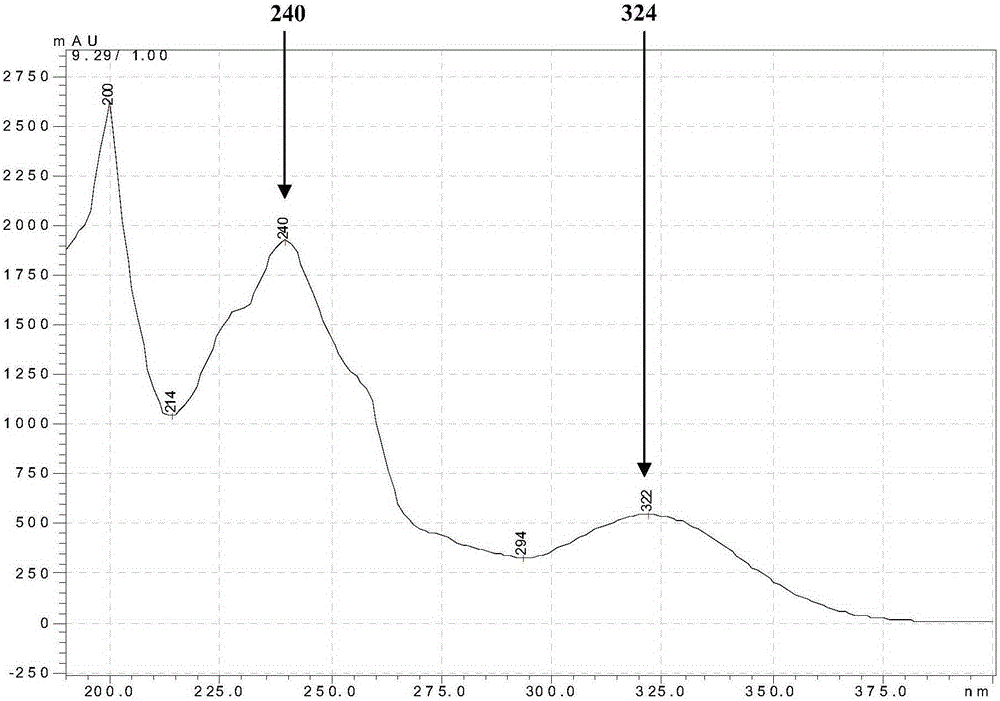
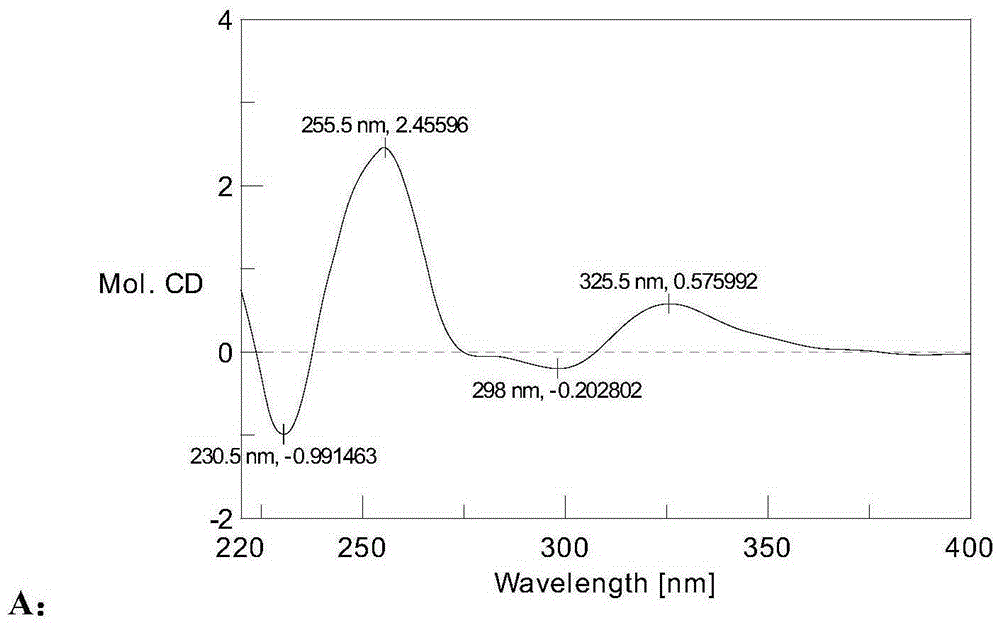
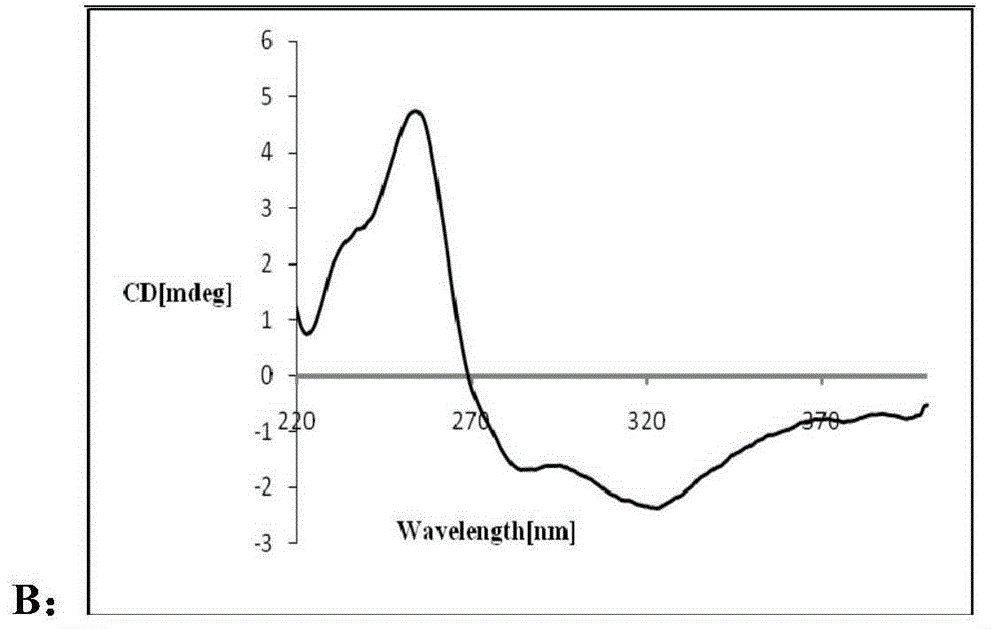
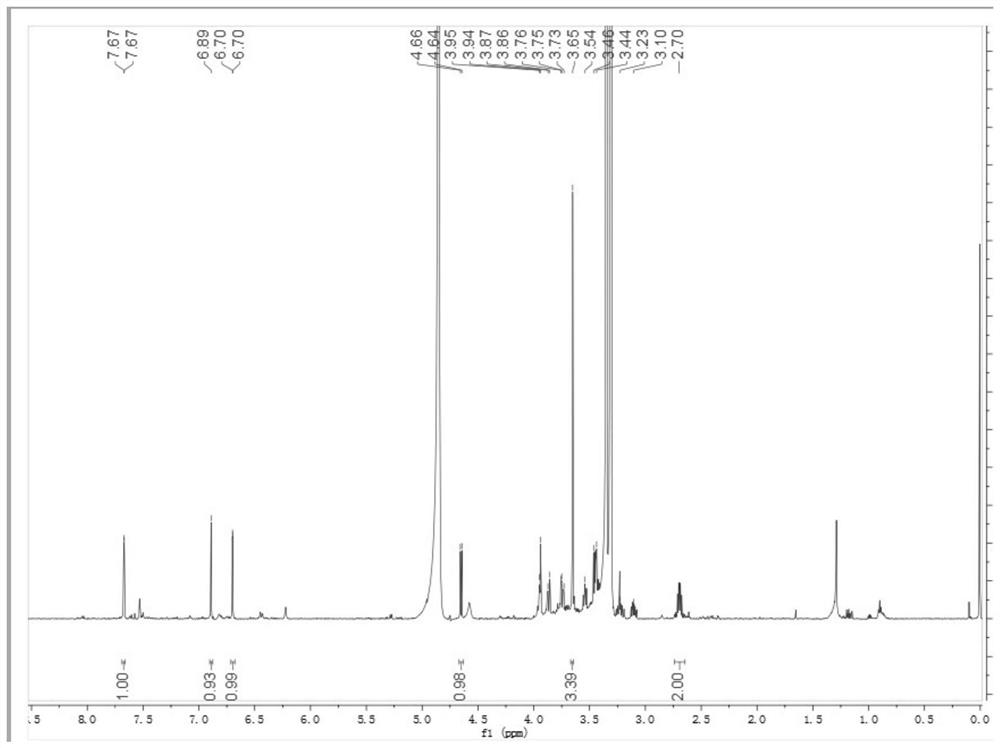
Patents
Literature
46 results about "Enzyme Inhibitor Drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of betulinic acid in preparing glycosidase inhibitor medicine
InactiveCN101416971ALow costRich sourcesOrganic active ingredientsMetabolism disorderLupeolDrug excipient
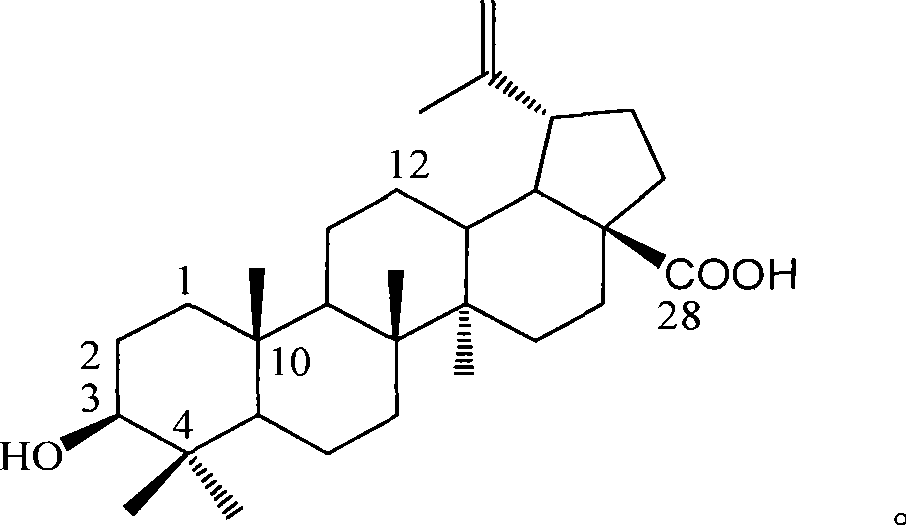
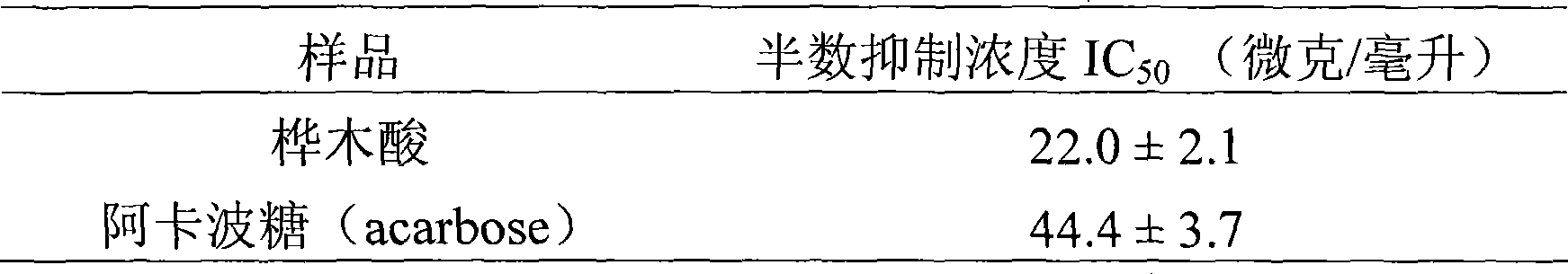
The invention provides the application of betulinic acid and salts thereof suitable for medical purpose in the preparation of a drug of a glycosidase inhibitor. The compound of the invention is the compound of lupeol type pentacyclic triterpene acid obtained by separating lagerstroemia plants. Pharmacological tests prove that the compound has obvious effect in inhibiting Alpha-glucosidase, the inhibitory activity of which exceeds acarbose 100% for initial therapy. Therefore, drug compositions made from the compound or the salts thereof suitable for medical purpose and drug excipients or carriers allowable for preparation can be applied to the preparation of drugs for preventing or treating Type II Diabetes, namely, noninsulin-dependent diabetes mellitus. The constitutional formula shown in formula (1) of the invention is shown as above.
Owner:ZHEJIANG UNIV
Medical prosthetic devices and implants having improved biocompatibility
InactiveUS20060155384A1Electrolytic inorganic material coatingBone implantZirconium hydrideEnzyme Inhibitor Drugs
A medical prosthetic device or medical implant containing a metal material (A) selected from the group consisting of titanium or an alloy thereof, zirconium or an alloy thereof, tantalum or an alloy thereof, hafnium or an alloy thereof, niobium or an alloy thereof and a chromium-vanadium alloy, wherein surface parts of the metal material (A) are coated with a layer of a corresponding hydride material (B) selected from titanium hydride, zirconium hydride, tantalum hydride, hafnium hydride, niobium hydride and chromium and / or vanadium hydride, respectively, said device or implant being characterised in that the layer of hydride material (B) comprises one or more biomolecule substances (C) associated therewith. The device or implant exhibits improved biocompatibility. The metal material (A) is preferably titanium. The biomolecule substance (C) may be selected from the following types of substances: Natural or recombinant bio-adhesives; natural or recombinant cell attachment factors; natural, recombinant or synthetic biopolymers; natural or recombinant blood proteins; natural or recombinant enzymes; natural or recombinant extracellular matrix proteins; natural or synthetic extracellular matrix biomolecules; natural or recombinant growth factors and hormones; natural, recombinant or synthetic peptide hormones; natural, recombinant or synthetic deoxyribonucleic acids; natural, recombinant or synthetic ribonucleic acids; natural or recombinant receptors; enzyme inhibitors; drugs; biologically active anions and cations; vitamins; adenosine monophosphate (AMP), adenosine diphosphate (ADP) or adenosine triphosphate (A TP); marker biomolecules; amino acids; fatty acids; nucleotides (RNA and DNA bases); and sugars.
Owner:NUMERICAL TECH INC
Penicillazine derivative, and preparation and use thereof
ActiveCN101376655AHas enzyme inhibitory activityInhibits alpha-glucosidaseOrganic active ingredientsOrganic chemistryChromatographic separationEnzyme Inhibitor Drugs
The invention provides a penicillazine derivative and the preparation method and the application thereof. The preparation method comprise the following steps: culturing strains of marine fungi in a culture medium, fermentation culturing the fungi in a fermentation culture medium, filtering the obtained fermented solution to remove thalli; heating to concentrate the filtrate and extracting with an organic solvent; carrying out the chromatographic separation and concentrating the eluate to obtain penicillazine; and adding an alkylation or acylation reagent and reacting to obtain the penicillazine derivative. The penicillazine derivative derived from the marine fungihas an enzyme inhibiting activity, can effectively inhibit Alpha-glucosidase, and can be applied for developing enzyme inhibiting pharmaceuticals. The raw material can be produced in a large scale, and the application prospect is wide.
Owner:OCEAN UNIV OF CHINA
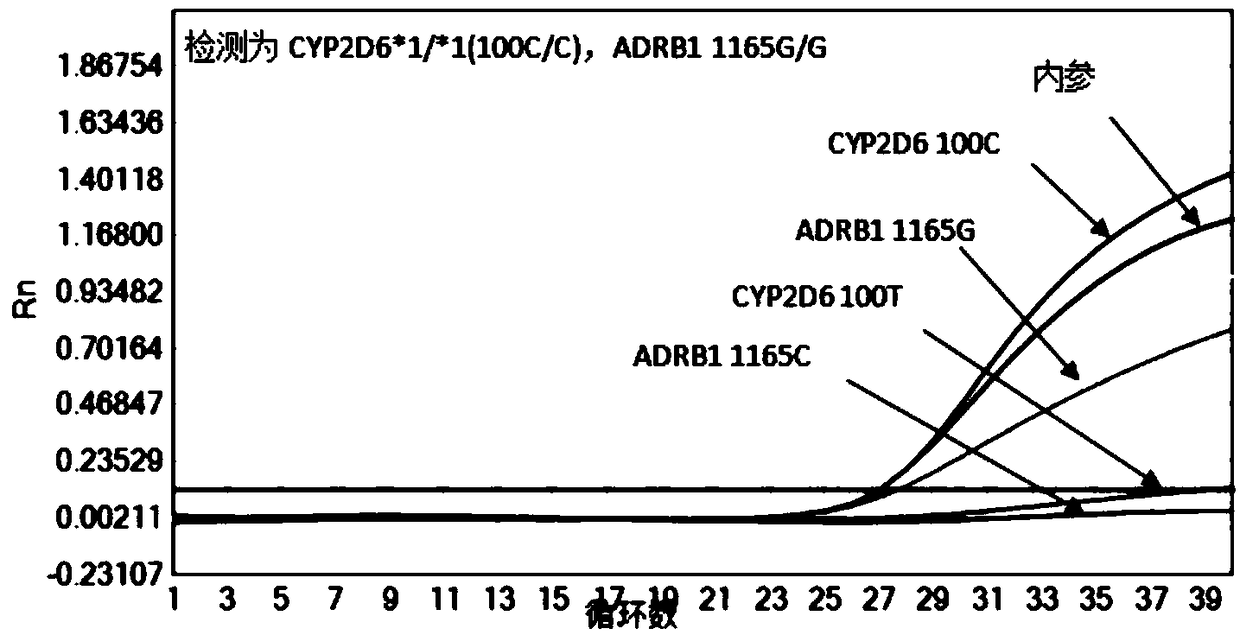
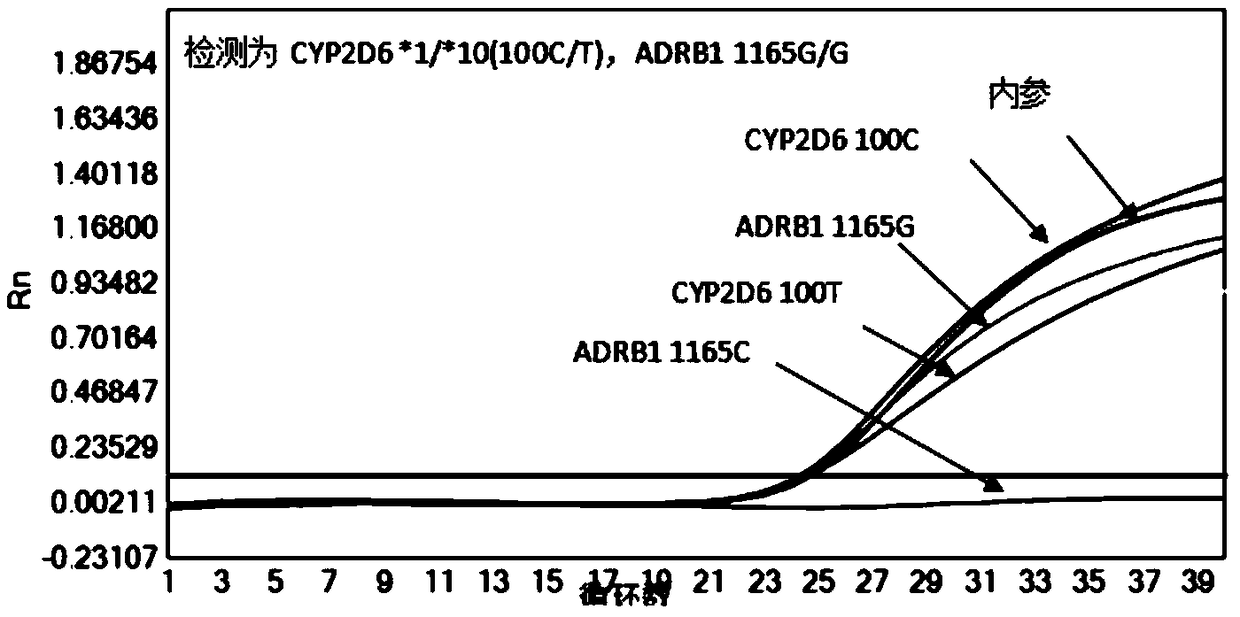
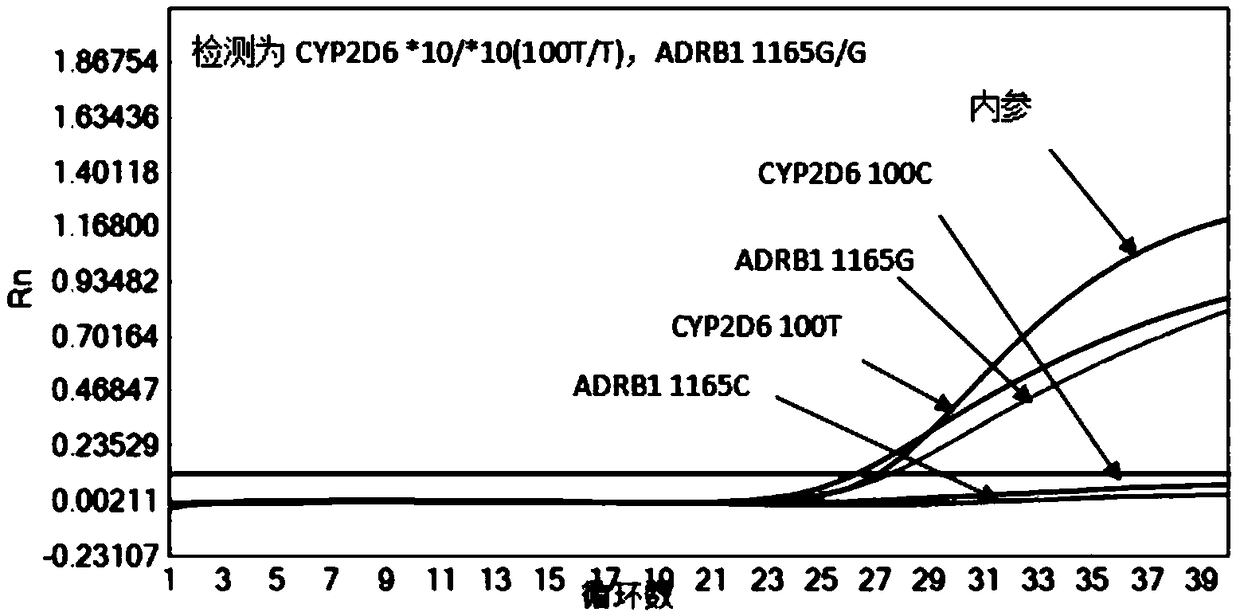
Reagent kit and method for quickly detecting polymorphism of hypertension individualized medication genes
InactiveCN109355368AEasy to operateStable storageMicrobiological testing/measurementEnzyme Inhibitor DrugsAnti hypertension
The invention discloses a reagent kit and a method for quickly detecting the polymorphism of hypertension individualized medication genes, belongs to the technical field of gene detection, and relatesto clinical anti-hypertension medicine beta 1 receptor blocker, angiotensin II receptor 1 antagonist and angiotensin converting enzyme inhibitor medicine metabolism. The reagent kit comprises sampletreatment reagents, premixed and individual-portion separately packaged gene detection reagent PCR (polymerase chain reaction) liquid 1, PCR liquid 2, PCR liquid 3, positive reference substances and negative reference substances. The sample treatment reagents are used for quickly treating human whole-blood samples. The reagent kit and the method have the advantages that the polymorphism of the hypertension individualized medication genes can be conveniently, quickly and efficiently detected by the aid of the reagent kit and the method on the basis that the detection specificity and sensitivityare guaranteed, detection operation steps can be reduced, the detection reaction time can be shortened, the production cost and the detection cost can be reduced, and the reagent kit and the method are favorable for clinical detection and analysis application.
Owner:江苏美因康生物科技有限公司
Applications of four kaurane diterpene compounds in preparation of glycosidase inhibitor medicines
ActiveCN104083348APotent inhibitory activityInhibitory activityMetabolism disorderEster active ingredientsGlycosidase activityMonomer
The invention provides applications of four kaurane diterpene compounds in preparation of glycosidase inhibitor medicines. The four compounds capable of highly inhibiting alpha-glycosidase activity are natural compounds which are high in safety and capable of rapid natural degradation without residue in the environment. The four compounds are obtained by separation from wedelia trilobata, and other plant materials. The plant materials are rich in source. A preparation process is easy in operation. Monomers of the four compounds are stable and liable to store. The alpha-glucosidase inhibition activity of the four compounds is obviously higher than or equivalent to that of acarbose that is a clinic medicine. The four compounds are extremely likely developed into effective and safe alpha-glucosidase inhibitor medicines used for preventing and treating type II diabetes, and have good prospects.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Preparation method of spermacoce latifolia triterpenoids and application of spermacoce latifolia triterpenoid in preparation of glycosidase inhibitor medicine
InactiveCN104490894APotent alpha-glucosidase activityStrong preventionOrganic active ingredientsMetabolism disorderDisaccharidaseGlycosidase inhibitor
The invention provides a preparation method of spermacoce latifolia triterpenoids and an application of the spermacoce latifolia triterpenoids in preparation of a glycosidase inhibitor medicine. Six compounds for intensively inhibiting the activity of alpha-glycosidase provided by the invention are natural compounds which are high in safety, and can be naturally degraded rapidly without a residue in the environment. The compounds can be separated from plant materials such as spermacoce latifolia; the plant materials are abundant in source; and the preparation process is easy to operate. Monomers of the six compounds are relatively stable and easy to store; the alpha-glycosidase inhibiting activity is obviously higher than that of clinical medicine acarbose; and the compounds are extremely likely to be further developed into the effective and safe alpha-glycosidase inhibitor medicines for preventing and treating type 2 diabetes mellitus, and have relatively good prospects.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Novel 23-oleanolic acid compound as well as preparation method and application of compound in preparation of glycosidase inhibitor medicine
ActiveCN103739653AEnhanced inhibitory effectBroad application potentialOrganic active ingredientsMetabolism disorderDiabrezideMonomer
The invention discloses a compound 2-hydroxyl-3-carbonyl-23-oleanolic acid-1,4,12-triene-28-acid as well as a preparation method and application of the compound preparation of a glycosidase inhibitor medicine. An alpha-glucosidase inhibitor with high efficiency is extracted and separated from an akebia plant, a plant material is rich in source, an extraction preparation method is easy to operate, the plant can be used for a long time without being damaged when extraction is performed by adopting a plant fruit, and thus the economic benefit can be increased, and the environment friendliness is achieved; the monomeric compound is stable and easy to store. Pharmacological experiments prove that the inhibitory activity of the compound 2-hydroxyl-3-carbonyl-23-oleanolic acid-1,4,12-triene-28-acid is stronger than that of a first-grade diabetes drug acarbose by about 16 times, and thus the compound 2-hydroxyl-3-carbonyl-23-oleanolic acid-1,4,12-triene-28-acid can be expected to be further developed into a medicine for clinically treating type 2 diabetes mellitus and is good in prospect.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
New 23, 29-drop oleanolic acid compound, preparation method thereof and application in preparation of glucosidase inhibitor medicines
ActiveCN103739652AEnhanced inhibitory effectBroad application potentialOrganic active ingredientsMetabolism disorderEnzyme Inhibitor DrugsAklanonic acid
The invention discloses new 2-hydroxy-3-carbonyl-23, 29-dinorolean-1, 4, 12, 20 (30)-tetraene-28-acid, a preparation method thereof and an application in preparation of glucosidase inhibitor medicines. An alpha-glucosidase inhibitor with strong effects is extracted and separated from an akebia plant, and plant sources are rich. Furthermore, when fruit extraction is performed, the plant can not be damaged and can be utilized for a long time, so that economic benefits are improved, and the environment-friendly effect is further achieved. Pharmacological experiments show that the in-vitro alpha-glucosidase inhibition activity of the compound, namely 2-hydroxy-3-carbonyl-23, 29-dinorolean-1, 4, 12, 20 (30)-tetraene-28-acid is about 5 times of that of a first-line diabetes medicine, namely acarbose, so that the compound is expected to be used for developing medicines for preventing and treating type II diabetes and has good application and development potentials.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Preparation method of Plk (Polo-like kinase) inhibitor drug intermediate 7-amino-2,3-dihydrobenzofuran-4-formic acid
ActiveCN103664845ASimple processFew reaction stepsOrganic chemistryBenzoic acidEnzyme Inhibitor Drugs
The invention discloses a preparation method of Plk (Polo-like kinase) inhibitor drug intermediate 7-amino-2,3-dihydrobenzofuran-4-formic acid (I), which comprises the following steps: by using 3-hydroxy-4-nitrobenzoic acid as a starting material, firstly esterifying, then condensing the starting material with 2-halogeno acetaldehyde or 2-halogeno acetal under the effect of an alkali acid-binding agent to realize alkylation of hydroxyl, then performing Friedel-Crafts reaction under the catalysis of protonic acid or Lewis acid, performing cyclization to obtain 7-nitrobenzofuran-4-methyl formate, and finally performing catalytic and hydride reduction and hydrolysis to obtain high-yield 7-amino-2,3-dihydrobenzofuran-4-formic acid (I). According to the invention, the synthetic route is simplified and optimized, the reaction processes are reduced, the technology is simplified, the cost is lowered and the production yield is high, and the 7-amino-2,3-dihydrobenzofuran-4-formic acid can be produced in large scale to meet the usage requirements, and is suitable for industrial production.
Owner:湖南欧亚药业有限公司
Novel 2,3-dihydroxyl-30-noroleanolic acid as well as preparation method and application thereof in preparing glycosidase inhibitor medicament
ActiveCN103641882AEnhanced inhibitory effectBroad application potentialOrganic active ingredientsMetabolism disorderDiabrezideAlpha-glucosidase inhibitor
The invention discloses novel 2,3-dihydroxyl-30-noroleanolic acid as well as a preparation method thereof and application thereof in preparing a glycosidase inhibitor medicament. According to the novel 2,3-dihydroxyl-30-noroleanolic acid as well as a preparation method thereof and application thereof disclosed by the invention, a strong-effect alpha-glucosidase inhibitor is extracted and separated from akebia plants, and the plants are rich in resource. Moreover, when fruit extraction is adopted, the plants are utilized for a long time without being damaged, so that not only can economic benefits be improved, but also environmental friendliness can be achieved. A pharmacological experiment shows that a compound 2,3- dihydroxyl-23-aldehyde group-nor olean-12,20(29)-diene-28-acid has in-vitro alpha-glucosidase-inhibitory activity stronger than that of a first-line diabetes medicament acarbose, is expected to be further developed into a medicament for preventing and treating type II diabetes mellitus, and has good application and development potentials.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Alkaloid compounds, method for preparing same and applications as enzyme inhibitors
ActiveCN101307049AHas enzyme inhibitory activityInhibits human topoisomerase IOrganic active ingredientsOrganic chemistryChromatographic separationAcetic anhydride
The invention relates to an alkaloid compound, a method for preparing the alkaloid compound and an application of the alkaloid compound as enzyme inhibitor. During preparation, strain culture is first carried out to a marine fungus in a culture medium, and then fermentation culture is carried out to the fungus in a fermentation culture medium; obtained fermentation liquid is filtered to eliminate mycelium; filtrate is heated, concentrated and extracted through ethyl acetate; chromatographic separation is carried out, and after obtained eluent is concentrated, penicillazine is obtained; bromine or manganese dioxide is added to a solution dissolving the Penicillazine, and then Penicillazines 1 and 2 are respectively obtained after reaction; or acetic anhydride is added to a solution dissolving the Penicillazine 2, and then Penicillazines 3 and 4 are obtained after reaction. An alkaloid derivative derived from the marine fungus can effectively inhibit human topoisomerase I, can be used for developing enzyme inhibitor medicine, and has raw materials that can be produced in large scale.
Owner:OCEAN UNIV OF CHINA
Chromones compound, preparing method thereof and chromones compound application in preparing antineoplastic and enzyme inhibitor medicines
ActiveCN103483354AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryEnzyme Inhibitor DrugsChromone
The invention discloses a chromones compound, a preparing method thereof and chromones compound application in preparing antineoplastic and enzyme inhibitor medicines. The sulfur-containing chromones compound shown in a formula (I) is strong in effect of inhibiting the growth of lung adenocarcinoma cells or histocyte lymphoma cells or leukemia cells or gastric carcinoma cells or acute lymphoblastic leukemia cells or acute myeloblastic leukemia cells or hepatoma carcinoma cells. The chromones compound inhibits JAK3, AuroraA and ABL. The chromones compound has good application prospects in preparing the antineoplastic and the enzyme inhibitor medicines.
Novel 2,3-dihydroxyl-30-noroleanolic acid as well as preparation method and application thereof in preparing glycosidase inhibitor medicament
ActiveCN103641882BRich sourcesHigh α-glucosidase inhibitory activityOrganic active ingredientsMetabolism disorderEnzyme Inhibitor DrugsAldehyde
The invention discloses novel 2,3-dihydroxyl-30-noroleanolic acid as well as a preparation method thereof and application thereof in preparing a glycosidase inhibitor medicament. According to the novel 2,3-dihydroxyl-30-noroleanolic acid as well as a preparation method thereof and application thereof disclosed by the invention, a strong-effect alpha-glucosidase inhibitor is extracted and separated from akebia plants, and the plants are rich in resource. Moreover, when fruit extraction is adopted, the plants are utilized for a long time without being damaged, so that not only can economic benefits be improved, but also environmental friendliness can be achieved. A pharmacological experiment shows that a compound 2,3- dihydroxyl-23-aldehyde group-nor olean-12,20(29)-diene-28-acid has in-vitro alpha-glucosidase-inhibitory activity stronger than that of a first-line diabetes medicament acarbose, is expected to be further developed into a medicament for preventing and treating type II diabetes mellitus, and has good application and development potentials.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
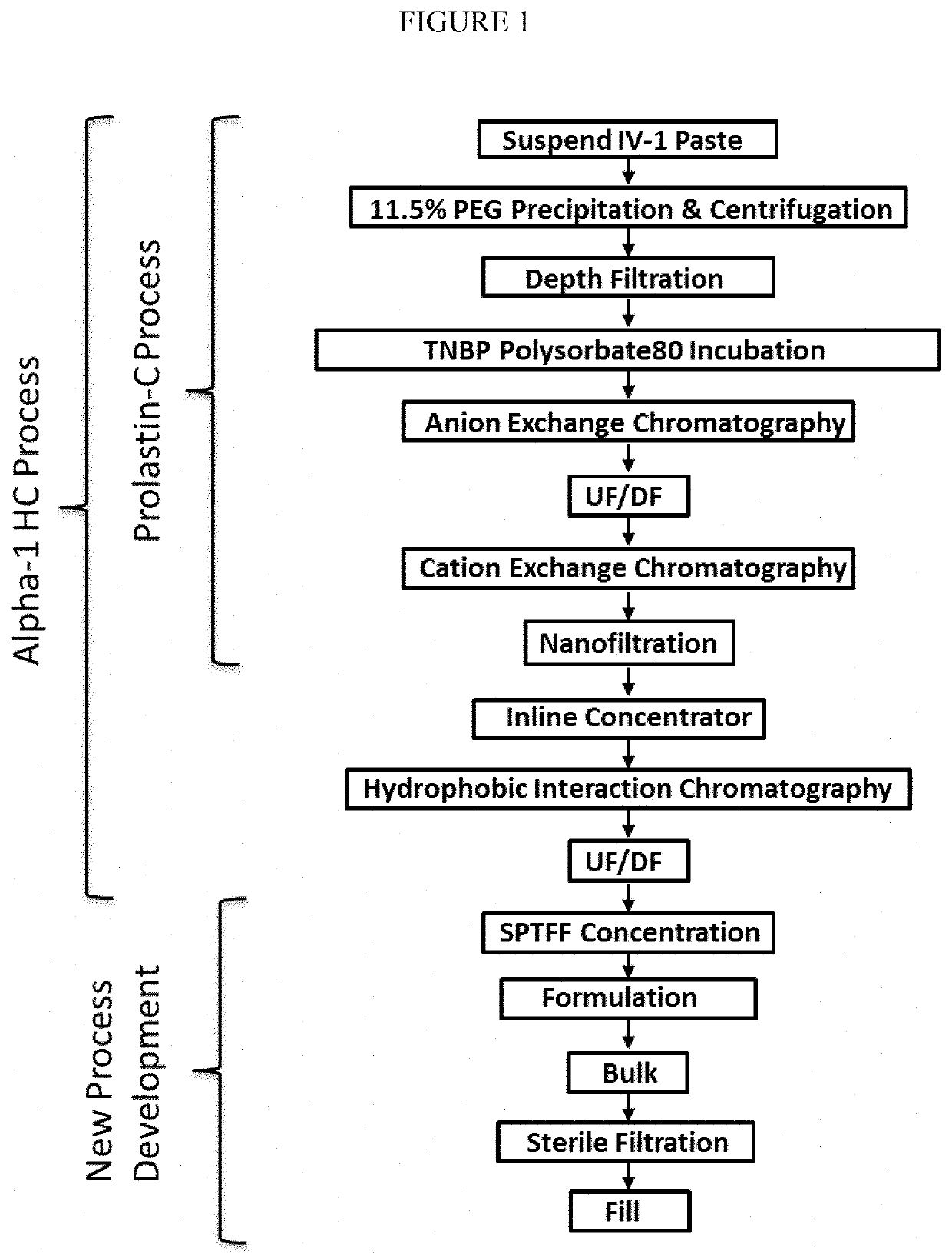
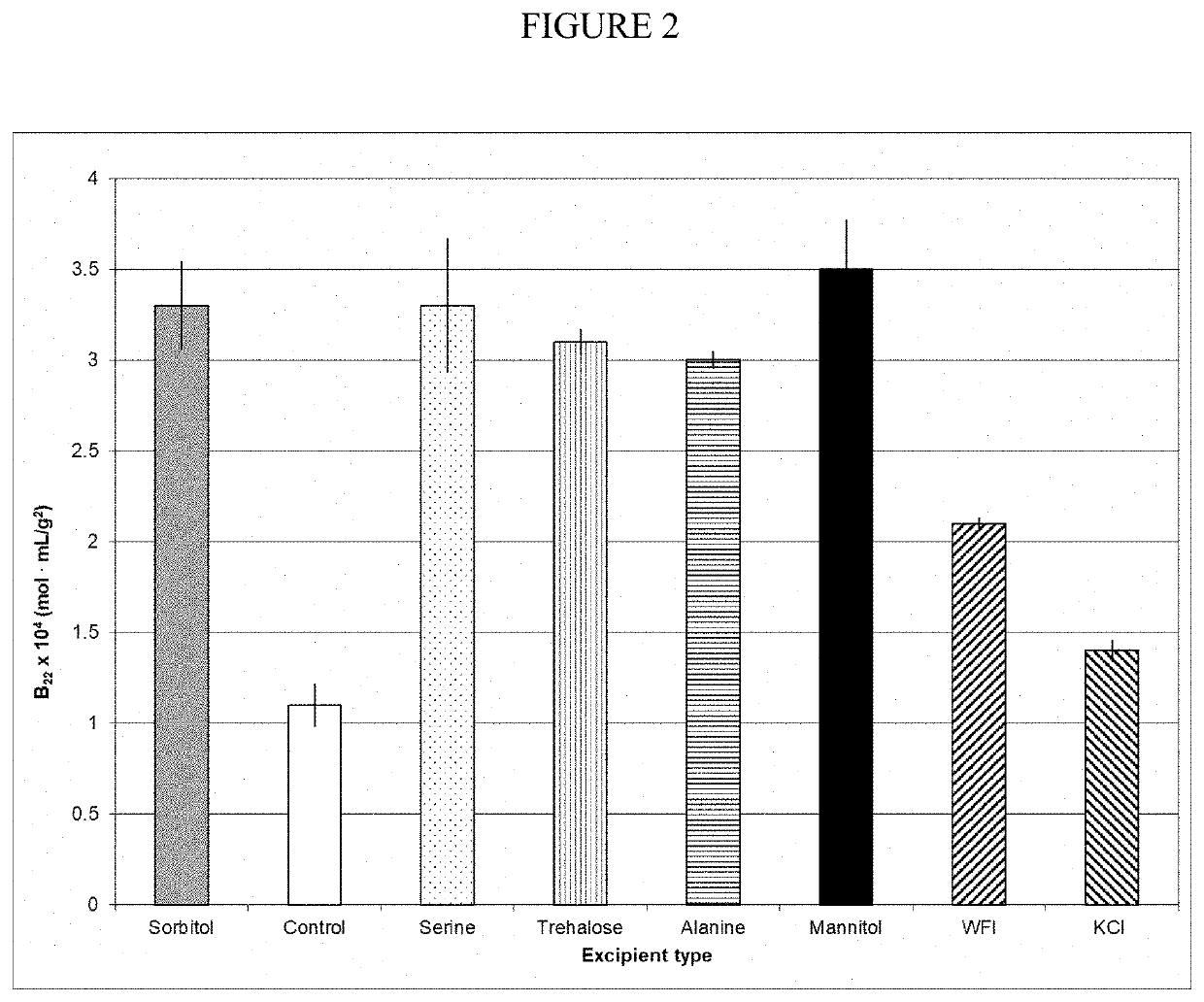
Composition comprising highly-concentrated alpha1 proteinase inhibitor and method for obtaining thereof
ActiveUS20200038494A1Cause immediate hypersensitive reactionsEasy to managePowder deliveryPeptide/protein ingredientsAlpha1-proteinase inhibitorEnzyme Inhibitor Drugs
Compositions include highly-concentrated Alpha-1 Proteinase Inhibitor (A1PI) in a concentration greater than or equal to 100 mg / ml. Pharmaceutical compositions can be prepared from these compositions. The pharmaceutical compositions can be suitable for subcutaneous administration. The highly-concentrated A1PI solutions can be obtained by single-pass tangential flow filtration (SPTFF).
Owner:GRIFOLS WORLDWIDE OPERATIONS
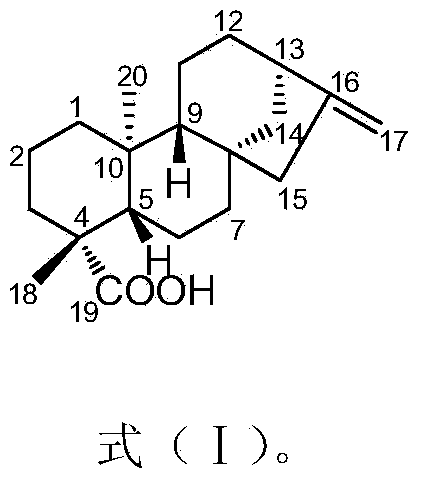
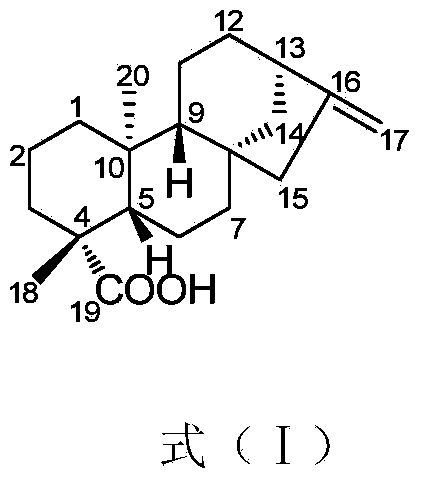
Applications of compound ent-kaur-16-en-19-oic acid in preparation of glycosidase inhibitor medicines
InactiveCN104083347AAbundant sources of materialsEasy to prepareMetabolism disorderAnhydride/acid/halide active ingredientsDrugAlglucerase
The invention discloses applications of a compound ent-kaur-16-en-19-oic acid in preparation of glycosidase inhibitor medicines. The compound ent-kaur-16-en-19-oic acid has high alpha-glucosidase inhibition activity, and the alpha-glucosidase inhibition activity of the compound is even higher than that of acarbose that is a first-line medicine for diabetes, and therefore the compound can be used for development and preparation of potential medicine candidates used for preventing and treating physiological changes or diseases caused by or related to alpha-glucosidase, wherein the physiological changes or diseases caused by or related to the alpha-glucosidase include but is not limited to type II diabetes.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
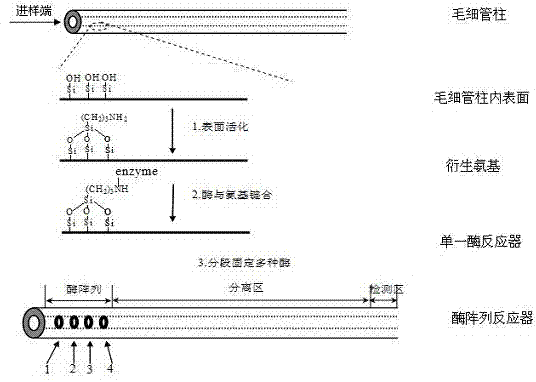
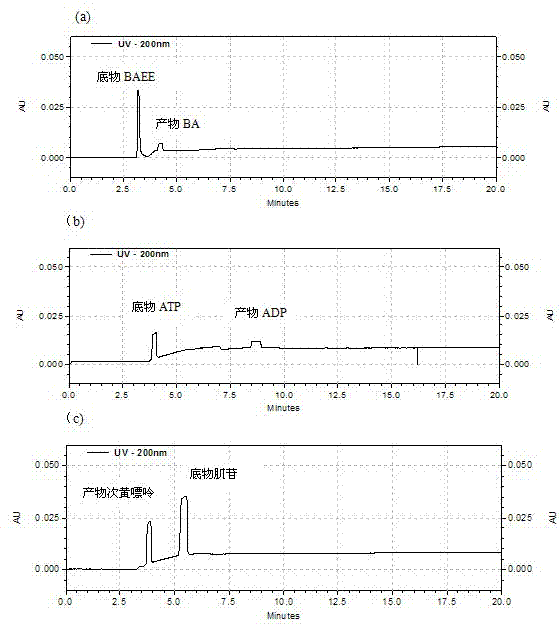
Capillary enzyme array micro-reactor and its application
InactiveCN103091385AImprove throughputEnable Interaction StudiesMaterial analysis by electric/magnetic meansEnzyme Inhibitor DrugsHigh flux
The invention discloses a capillary enzyme array micro-reactor and its application. The enzyme array micro-reactor is formed through constructing a plurality of enzymes in a capillary in addressable section based on a covalent fixing technology in the invention. The capillary enzyme array micro-reactor integrates a plurality of types of enzyme reactors, and can simultaneously realize the interacting high-flux analysis of the plurality of enzymes within a unit time, so the research period is substantially shortened. The capillary enzyme array micro-reactor has the advantages of simple method, low cost, high analysis efficiency, and easy automation, and has great scientific research values and economic benefits in the high-flux screening and the like of enzyme inhibitors and medicines.
Owner:MINZU UNIVERSITY OF CHINA
Abiraterone precursor compound as well as preparation method and application thereof
PendingCN113527401AGood water solubilityImprove bioavailabilityOrganic active ingredientsSteroidsDiseaseEnzyme Inhibitor Drugs
The invention discloses a precursor compound of a novel abiraterone medicine as well as a preparation method and application thereof. The structure of the compound is shown as a formula I in the specification. The invention further provides a preparation method of the compound shown in the formula I, hydrochloride of the compound shown in the formula I, a preparation method of the compound and hydrochloride and application of the compound and hydrochloride in the preparation of CYP17 enzyme inhibitor drugs. The compound provided by the invention provides improved oral bio-availability and pharmacokinetic characteristics, can be used as a human body CYP17 enzyme inhibitor, and can be used for treating urogenital or androgen-related diseases, such as cancers or other androgen-related diseases, including prostatic cancer, breast cancer, prostatic hyperplasia and the like.
Owner:SUZHOU AIHE PHARM TECH CO LTD
Alkaloid compounds, method for preparing same and applications as enzyme inhibitors
ActiveCN101307049BHas enzyme inhibitory activityInhibits human topoisomerase IOrganic active ingredientsOrganic chemistryChromatographic separationEnzyme Inhibitor Drugs
The invention relates to an alkaloid compound, a method for preparing the alkaloid compound and an application of the alkaloid compound as enzyme inhibitor. During preparation, strain culture is first carried out to a marine fungus in a culture medium, and then fermentation culture is carried out to the fungus in a fermentation culture medium; obtained fermentation liquid is filtered to eliminatemycelium; filtrate is heated, concentrated and extracted through ethyl acetate; chromatographic separation is carried out, and after obtained eluent is concentrated, penicillazine is obtained; bromine or manganese dioxide is added to a solution dissolving the Penicillazine, and then Penicillazines 1 and 2 are respectively obtained after reaction; or acetic anhydride is added to a solution dissolving the Penicillazine 2, and then Penicillazines 3 and 4 are obtained after reaction. An alkaloid derivative derived from the marine fungus can effectively inhibit human topoisomerase I, can be used for developing enzyme inhibitor medicine, and has raw materials that can be produced in large scale.
Owner:OCEAN UNIV OF CHINA
29-noroleananoic acid compound and its preparation method and application in the preparation of glycosidase inhibitor medicine
ActiveCN103613632BRich sourcesGood prospects for marketizationMetabolism disorderSteroidsEnzyme Inhibitor DrugsAklanonic acid
The invention discloses a compound 2,3,20-trihydroxy-29-noroleanolic acid, as well as a preparation method and application thereof in preparing glycosidase inhibitor medicines. According to the preparation method, a powerful alpha-glycosidase inhibitor is extracted and separated from akebia plants, the plant material is rich in source, the extracting preparation method is easy to operate, and the plant cannot be destroyed and can be utilized for a long time when the plant fruits are adopted for extraction, so that the economical benefit can be increased, and the environment is protected. The monomeric compound is stable and easy to store. Pharmacological experiments indicate that the activity of the alpha-glycosidase inhibitor of the compound 2,3,20-trihydroxy-29-noroleana-12-ene-28-oic acid is stronger than that of the first line diabetes drug acarbose, and the compound is expected to be developed into a medicine for treating II-type diabetes in clinical application, and has good prospect.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
23-Norursane triterpene compound and its preparation method and application in the preparation of glycosidase inhibitor drugs
ActiveCN112028963BStrong preventionPowerful therapeuticOrganic active ingredientsMetabolism disorderEnzyme Inhibitor DrugsGlycosidase inhibitor
The invention discloses 23-norursane triterpene compound, its preparation method and its application in the preparation of glycosidase inhibitor drugs. The present invention separates and obtains the 23-norursane triterpene compound represented by the formula I with strong inhibitory activity of α-glucosidase from Akebia trifoliate, which has low potential toxic and side effects, rich source of plant materials, and when plant fruit is used for extraction It can also make the plant itself not too damaged and can be used for a long time, and the monomer compound is stable and easy to store, and its activity of inhibiting α-glucosidase is significantly stronger than that of clinical drug acarbose, which is likely to be further developed into an effective and safe The α-glucosidase inhibitor drugs for the prevention and treatment of type Ⅱ diabetes have good prospects.
Owner:GUANGDONG ACAD OF FORESTRY
Drug-Coated Balloon Controllable In Drug Metabolism And Preparation Method Therefor
PendingUS20220288358A1Lower metabolismReduce harmBalloon catheterCoatingsDrug metabolismEnzyme Inhibitor Drugs
The present invention provides a preparation method for a drug-coated balloon controllable in drug metabolism. A drug receptor protein inhibitor is mixed with drugs, water and ethanol to obtain a medicinal liquid, the medicinal liquid is sprayed onto the surface of a balloon back and forth by means of an ultrasonic spraying device, and drying is performed so as to prepare the drug-coated balloon. The drug receptor protein inhibitor can also be replaced with other agents such as a drug metabolism isoenzyme inhibitor, a drug metabolism isoenzyme inducer, or a mixture of the drug receptor protein inhibitor and the drug metabolism isoenzyme inhibitor for preparing the medicinal liquid, and a drug metabolic cycle of the drug-coated balloon can be further adjusted and controlled by adjusting the ratio of the addition quantity of the drug receptor protein inhibitor or the other agents to the drugs. The drug-coated balloon prepared by the method has the functions that effective controllable drug metabolism can be implemented without destroying the structure of an intima, the drug metabolism can be implemented to achieve the effect of drug treatment at an early stage of using the drug-coated balloon, and the effect period of the drugs can be prolonged by adjusting the ratio.
Owner:SHANGHAI HEARTCARE MEDICAL TECH CORP LTD
A kind of method utilizing sphingomonas to split hepatitis C ns3 enzyme inhibitor medicine intermediate
ActiveCN105861585BHigh selective resolution activityReduce dosageBacteriaMicroorganism based processesEnzyme Inhibitor DrugsCyclopropane
Owner:BEIJING UNIV OF CHEM TECH
Furocoumarin having DPPIV enzyme inhibition activity and preparation method of furocoumarin having DPPIV enzyme inhibition activity
ActiveCN110804079AGood inhibitory effectGood DPPIV enzyme inhibitory activityOrganic active ingredientsSugar derivativesFuranEnzyme Inhibitor Drugs
The invention provides furocoumarin having DPPIV enzyme inhibition activity and a preparation method of the furocoumarin having DPPIV enzyme inhibition activity. The structural formula of a furocoumarin type compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is as shown in a formula (I), and the furocoumarin type compound is separated from dry products or fresh products of fruits, roots, stems, branches or leaves of clausena lansium. In vitro pharmacological experiment confirms the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate has significant inhibition effects on DPPIV enzymes, the IC50 value of the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is 117+ / -8.7[mu]M, which illustrates that the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate has favorable DPPIV enzyme inhibition activity. The furocoumarin type compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is hopeful to be used as a lead compound for developing a novel DPPIV enzyme inhibitor medicine.
Owner:SERICULTURE & AGRI FOOD RES INST GUANGDONG ACAD OF AGRI SCI
The application of the active part of the cornflower inflorescence as a drug for the preparation of 5α reductase inhibitors
ActiveCN107334801BEnhanced inhibitory effectObvious effectDermatological disorderPlant ingredientsEnzyme Inhibitor DrugsSeparation technology
Owner:新疆金海娜生物科技有限公司 +1
Use of a cyclohexenyl-dl-aspartic acid derivative in the quality control of neuraminidase inhibitor pharmaceutical preparations
ActiveCN111920794BQuality improvementLow incidence of adverse reactionsOrganic active ingredientsNervous disorderEnzyme Inhibitor DrugsDepressant
One aspect of the invention provides a use of a cyclohexenyl-DL-aspartic acid derivative in the quality control of neuraminidase inhibitor pharmaceutical preparations, the cyclohexenyl-DL-aspartic acid derivative For the specific definition of the neuraminidase inhibitor, see the instructions. HPLC and structural confirmation studies show that the commercially available original preparation of the neuraminidase inhibitor contains a small amount of the cyclohexenyl-DL-aspartic acid derivative, and the cyclohexenyl-DL-aspartic acid derivative Aspartic acid derivatives are one of the main causes of neuropsychiatric adverse reactions caused by the original preparation of the neuraminidase inhibitor, so controlling them is crucial to improving the quality of medicines and reducing the incidence of adverse reactions.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
A class of chromone compounds, their preparation method and their application in the preparation of anti-tumor and enzyme inhibitor drugs
ActiveCN103483354BEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryEnzyme Inhibitor DrugsOncology
The invention discloses a chromones compound, a preparing method thereof and chromones compound application in preparing antineoplastic and enzyme inhibitor medicines. The sulfur-containing chromones compound shown in a formula (I) is strong in effect of inhibiting the growth of lung adenocarcinoma cells or histocyte lymphoma cells or leukemia cells or gastric carcinoma cells or acute lymphoblastic leukemia cells or acute myeloblastic leukemia cells or hepatoma carcinoma cells. The chromones compound inhibits JAK3, AuroraA and ABL. The chromones compound has good application prospects in preparing the antineoplastic and the enzyme inhibitor medicines.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
A kind of furocoumarin with dppiv enzyme inhibitory activity and preparation method thereof
ActiveCN110804079BStrong inhibitory activityPreparation conditions are easy to controlOrganic active ingredientsSugar derivativesFuranEnzyme Inhibitor Drugs
The invention provides furocoumarin having DPPIV enzyme inhibition activity and a preparation method of the furocoumarin having DPPIV enzyme inhibition activity. The structural formula of a furocoumarin type compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is as shown in a formula (I), and the furocoumarin type compound is separated from dry products or fresh products of fruits, roots, stems, branches or leaves of clausena lansium. In vitro pharmacological experiment confirms the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate has significant inhibition effects on DPPIV enzymes, the IC50 value of the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is 117+ / -8.7[mu]M, which illustrates that the compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate has favorable DPPIV enzyme inhibition activity. The furocoumarin type compound 6-beta-D-glucosyl-6,7-dihydroxy-5-benzfuran methyl propionate is hopeful to be used as a lead compound for developing a novel DPPIV enzyme inhibitor medicine.
Owner:SERICULTURE & AGRI FOOD RES INST GUANGDONG ACAD OF AGRI SCI
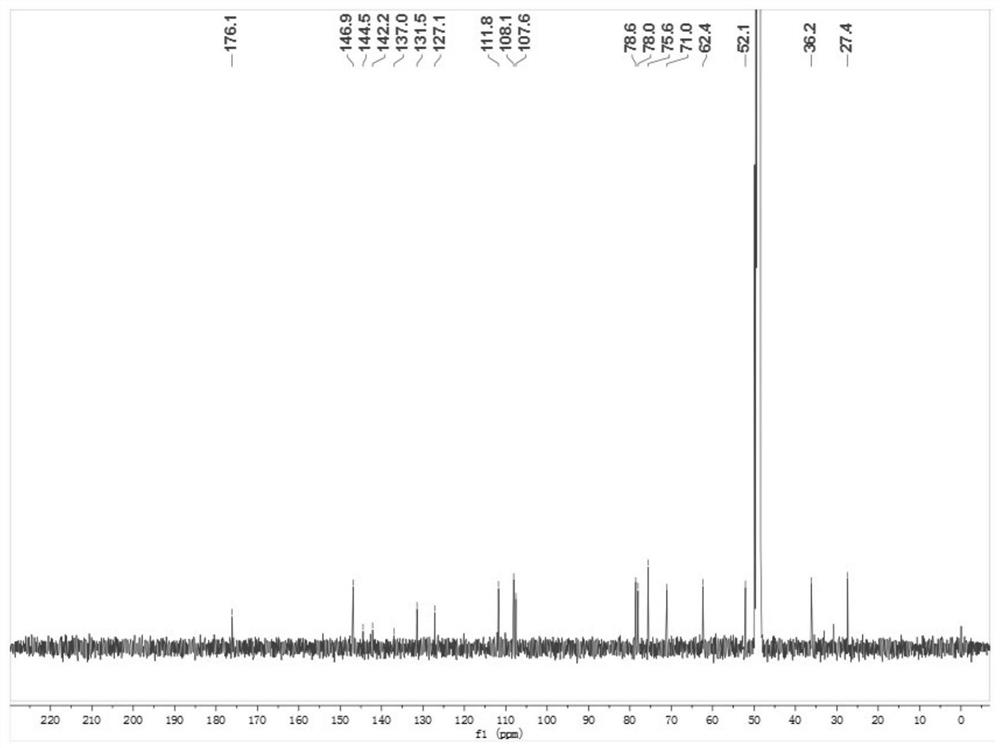
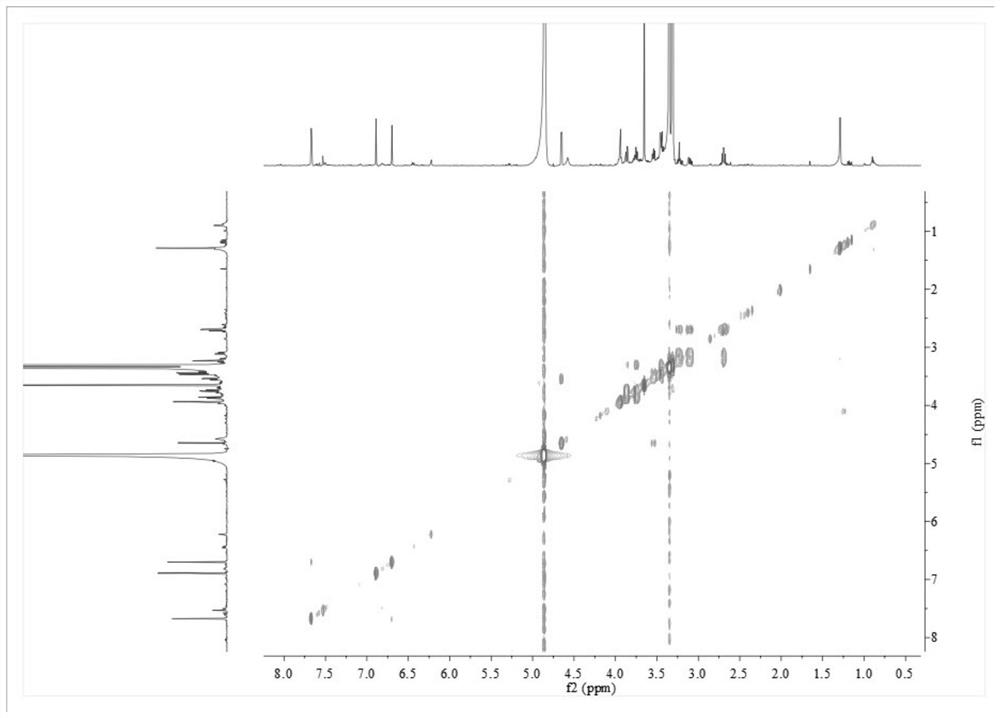
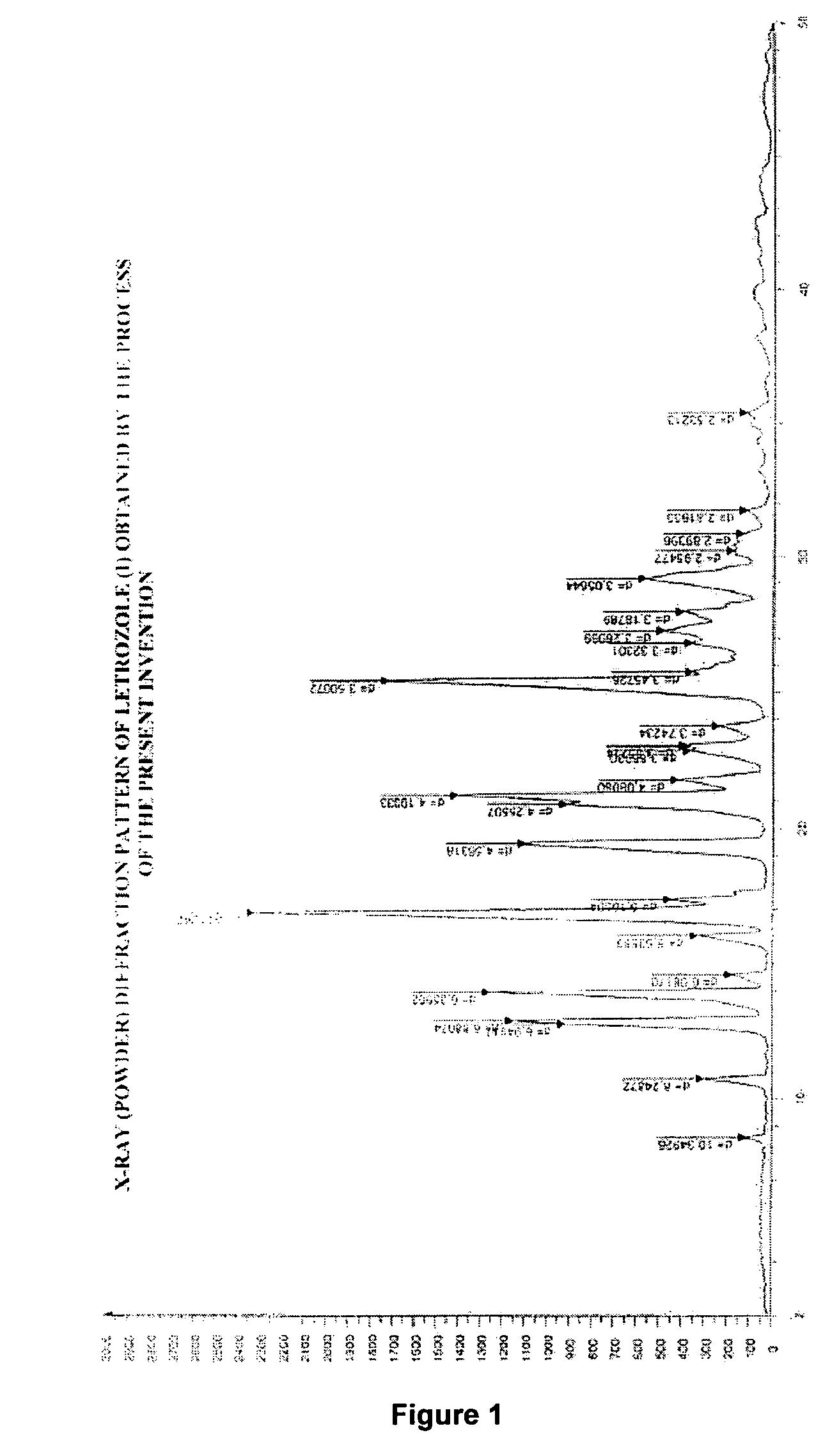
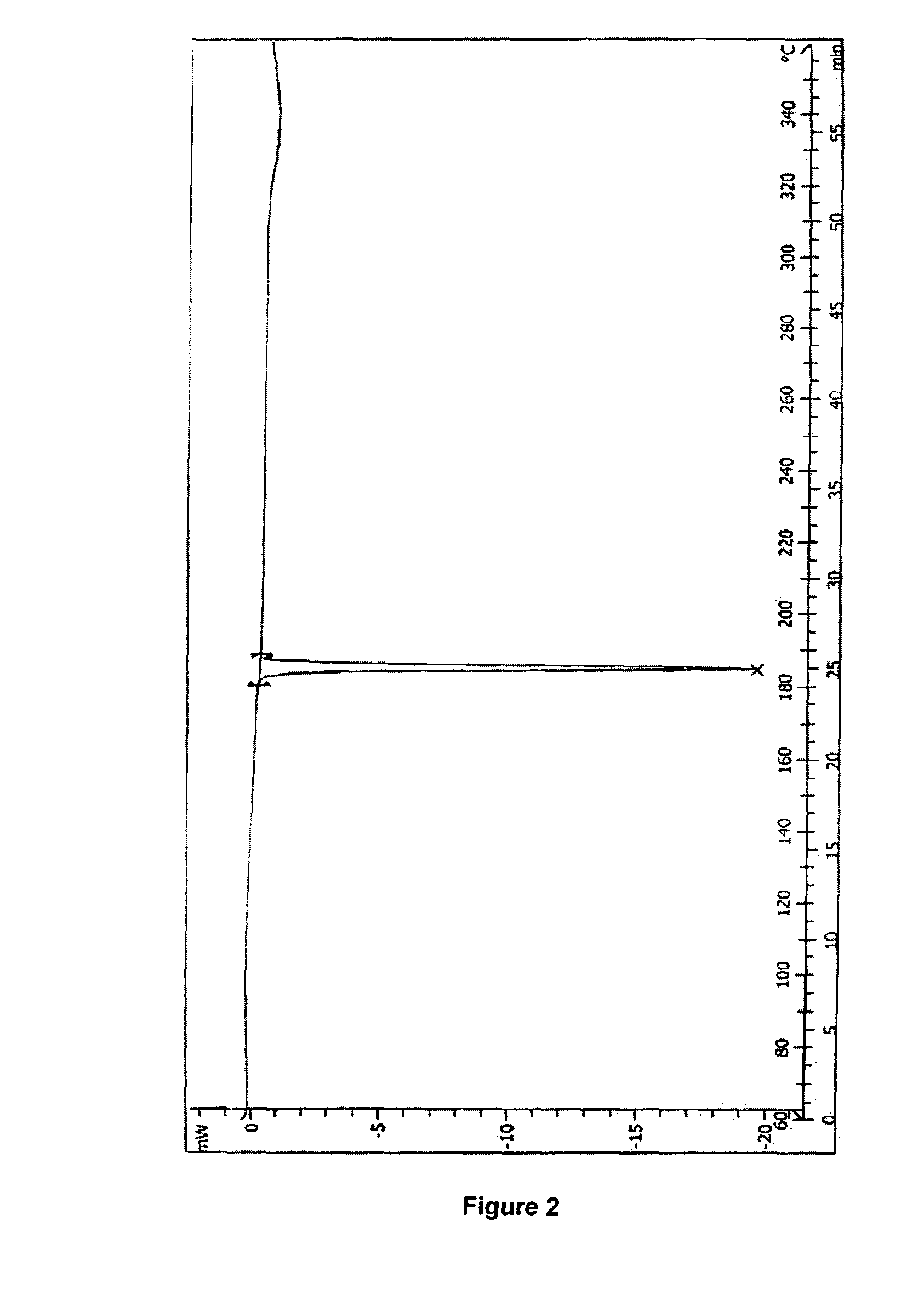
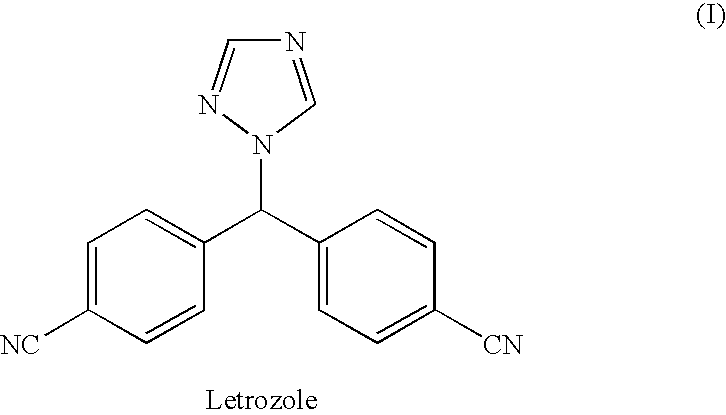
Process for preparation of letrozole and its intermediates
InactiveUS8198460B2Simple, convenient, economical and industrially viableHigh purityOrganic chemistryAntineoplastic agentsEnzyme Inhibitor DrugsAromatase inhibitor
The present invention relates to an improved process for preparation of the non-steroidal aromatase inhibitor drug, Letrozole of formula (I) and its intermediates, 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile of formula (IV) and 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile hydrochloride of formula (VII), all having a purity of ≧99%, which is simple, convenient, economical, does not use hazardous chemicals and industrially viable.
Owner:FRESENIUS KABI ONCOLOGY LTD
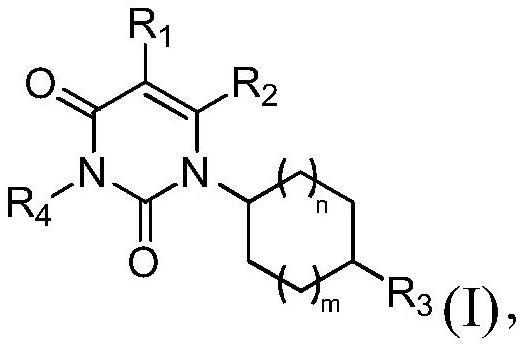
Pyrimidone compounds as chymase inhibitors and their applications
ActiveCN113811530BHigh activityExcellent PK propertiesOrganic active ingredientsOrganic chemistryEnzyme Inhibitor DrugsChymase
A class of compounds having a pyrimidone structure as a chymase inhibitor drug, specifically discloses the compound represented by formula (I), its pharmaceutically acceptable salts and isomers thereof, and pharmaceutical compositions containing them .
Owner:MEDSHINE DISCOVERY INC
Applications of cornflower inflorescence active part in serving as medicine for preparing 5-alpha reductase inhibitor
ActiveCN107334801AEnhanced inhibitory effectObvious effectDermatological disorderPlant ingredients5 Alpha-Reductase InhibitorSeparation technology
The invention relates to the technical field of 5-alpha reductase inhibitors, and provides applications of a cornflower inflorescence active part in serving as a medicine for preparing the 5-alpha reductase inhibitor. According to the technical scheme, the cornflower inflorescence active part is further refined through a modern separation technology and an activity research method, the cornflower inflorescence active part has a relatively good inhibition effect for the activity of the 5-alpha reductase, and meanwhile, the cornflower inflorescence active part adopted in the invention is a pure green plant extract, thus being safe and non-toxic and being remarkable in effect in inhibiting the activity of the 5-alpha reductase.
Owner:新疆金海娜生物科技有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com