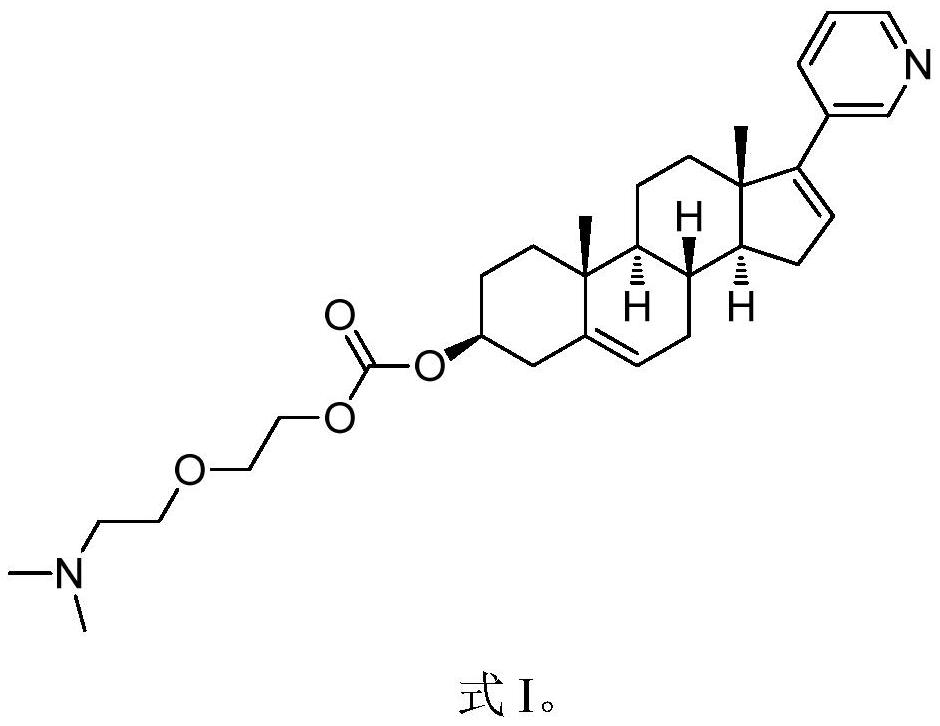
Abiraterone precursor compound as well as preparation method and application thereof
A compound and drug technology, applied in the field of abiraterone precursor compounds and their preparation, can solve the problems of stimulating tumor growth, unable to inhibit androgen, etc., and achieve the effects of increasing AUC, excellent water solubility, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 compound LHY-AB614
[0045]
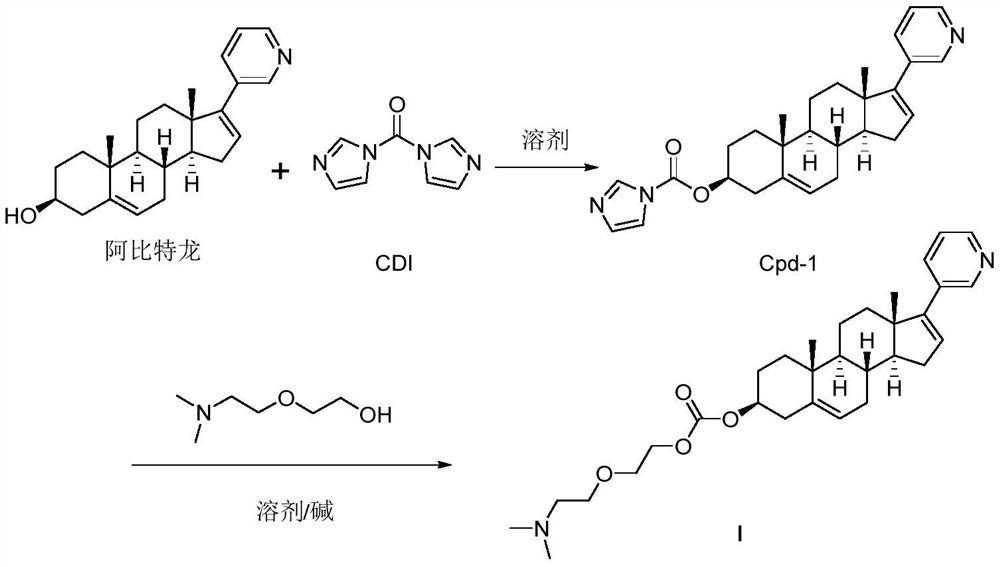
[0046] Take a dry 500ml round bottom flask, add abiraterone (10g, 28.6mmol, 1.0eq), and add 150mL of CH 2 Cl 2 Dissolve, add CDI (14g, 85.8mmol, 3.0eq) and react at room temperature. After 8 hours, the reaction was detected by TLC (PE:EA=1:1), and the raw materials disappeared completely. Concentrate the reaction solution, add 100 mL of dry THF, stir at room temperature for 30 min, filter with suction, and wash the filter cake with dry THF (50 mL*3). The solid was collected and dried to obtain compound Cpd-1 (10 g, 22.6 mmol) with a yield of 79%.
[0047] Take a dry 250mL round bottom flask, add compound Cpd-1 (10g, 22.6mmol, 1.0eq) and KOH (250mg, 4.5mmol, 0.2eq), add 100mL toluene, and finally add 2-[2-(dimethylamino ) Ethoxyl] ethanol (4.1mL, 29.4mmol, 1.3eq), react at 60°C overnight, TLC detects the reaction, and the raw material disappears completely. The solvent was spin-dried, and the resulting s...
Embodiment 2
[0050] The synthesis of embodiment 2 compound LHY-AB615
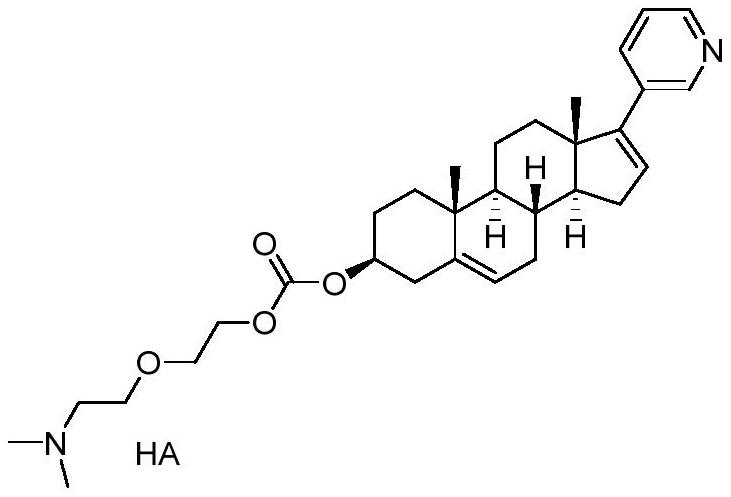
[0051] Dissolve formula I (1g) in 10mL ethyl acetate at room temperature, add an equivalent amount of phosphoric acid, after the reaction is complete, add 20mL petroleum ether, and the product LHY-AB615 is precipitated. Suction filtration and drying to obtain the crude product of LHY-AB615.
[0052] At room temperature, dissolve the crude product of LHY-AB615 (1g) in 1.2mL of methanol, slowly add 25mL of tetrahydrofuran dropwise, stir vigorously at room temperature, a white solid is precipitated, and the filter cake is dried after suction filtration to obtain relatively pure LHY-AB615 (820mg, purity 99.5%).
Embodiment 3
[0053] The synthesis of embodiment 3 compound LHY-AB616
[0054] Dissolve formula I (1 g) in 10 mL of ethyl acetate at room temperature, add an equivalent amount of methanesulfonic acid, after the reaction is complete, add 20 mL of petroleum ether, and the product LHY-AB616 is precipitated. Suction filtration and drying to obtain the crude product of LHY-AB616.
[0055] At room temperature, dissolve the crude product of LHY-AB616 (1g) in 1.2mL of methanol, slowly add 25mL of tetrahydrofuran dropwise, stir vigorously at room temperature, a white solid precipitates, and the filter cake is dried after suction filtration to obtain relatively pure LHY-AB616 (790mg, purity 99.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com