Pyrimidone compounds as chymase inhibitors and their applications
A compound and pharmaceutical technology, applied in the fields of drug combination, organic chemistry, medical preparations containing active ingredients, etc., can solve the problem that the PK property needs to be further improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
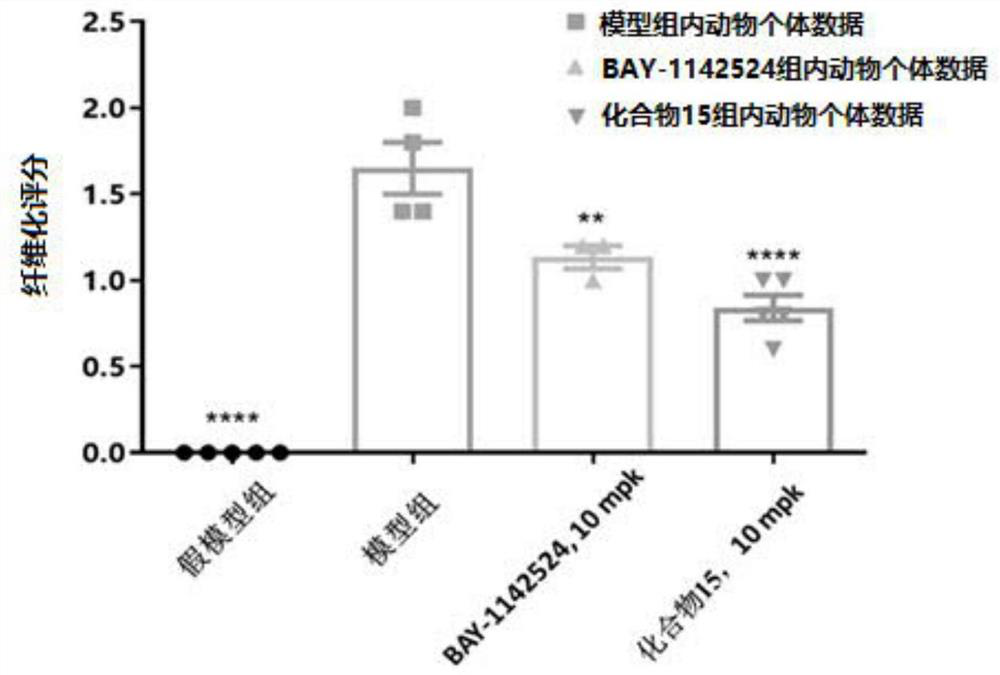
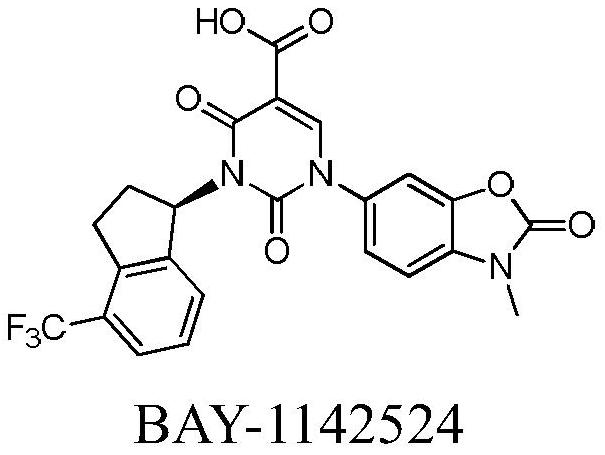
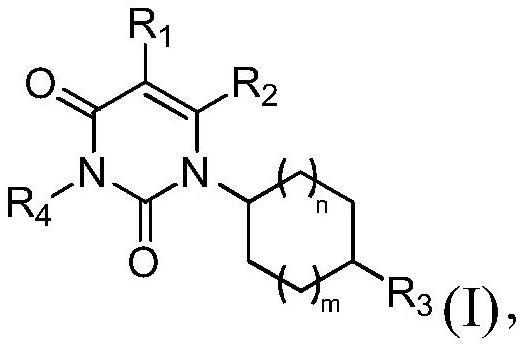
Image
Examples
Embodiment 1
[0109]
[0110] Step A: Compound 1-a (20g, 224.48mmol, 1eq) and monoethyl malonate (29.66g, 224.48mmol, 1eq) were dissolved in Ac at 75°C 2 O (400 mL) mixture was stirred for 4 h. The mixture was cooled to room temperature and concentrated under reduced pressure to obtain the crude product, which was purified by silica gel column chromatography (100-200 mesh, PE / EtOAc=20 / 1-3 / 1) to obtain 1-b.
[0111] Step B: Compound 1-b (21 g, 103.35 mmol, 1 eq) and triethyl orthoformate (15.32 g, 103.35 mmol, 17.19 mL, 1 eq) were dissolved in Ac at 140 °C 2 The O (25 mL) solution was stirred for 2 h. The mixture was cooled to room temperature and concentrated under reduced pressure to obtain crude product, which was purified by silica gel column chromatography (100-200 mesh, PE / EtOAc=10 / 1-1 / 1) to obtain 1-c.
[0112]
[0113] Step A: Chlorosulfonic acid (210.00 g, 1.80 mol, 120.00 mL, 13.11 eq) was added dropwise to compound 1-d (3-[2-(trifluoromethyl)phenyl]propionic acid) at 0°C ...
Embodiment 2
[0125]
[0126]
[0127] Aqueous HCl (2M, 27.08 mL, 4.46 eq) was added to AcOH (60 mL) in which compound 1 (6.5 g, 12.14 mmol, 1 eq) was dissolved at 15 °C, and the mixture was heated to 120 °C and stirred for 4 h. The mixture was cooled to room temperature and poured into H 2 O (200 mL), extract with EtOAc (100 mL×3), combine the organic phases and wash with saturated brine (300 mL×2), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the crude product of compound 2, which is then prepared by HPLC (column: Phenomenexluna c18 250 mm×100 mm×10 μm; mobile phase: [water (0.05% HCl)-acetonitrile]; acetonitrile %: 35%-65%, 20 min) and SFC (column: DAICEL CHIRALPAK AD (250 mm×30 mm, 10μm); mobile phase: [neutral-methanol]; methanol %: 35%-35%, 4.6min; 170min) purification to obtain compound 2-I (retention time: 2.780min) and compound 2-II (retention time: 3.718 min).
[0128] 2-I: 1 H NMR (400MHz, DMSO-d 6 ): δ=8.52(br...
Embodiment 3
[0131]
[0132] Step A: To a solution of compound 3-a (8g, 37.33mmol, 1eq) in DMF (80.00mL) at 15°C was added DIEA (4.82g, 37.33mmol, 6.50mL, 1eq) and 1-h (11.85g) , 37.33mmol, 1eq), the reaction solution was stirred for 12 hours. The reaction solution was poured into 600 mL of water, then diluted with 400 mL of EtOAc, the resulting mixture was extracted with EtOAc (400 mL×2), the organic phases were combined and washed with 400 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain the crude compound 3-b.
[0133] Step B: To a solution of compound 3-b (13.3 g, 31.92 mmol, 1 eq) in THF (60.00 mL) at 15 °C, a solution of tetrabutylammonium fluoride in THF (1 M, 63.85 mL, 2 eq) was added to react The solution was stirred at 15°C for 2 hours. The reaction solution was diluted with 50 mL of water and extracted with EtOAc (50 mL×2). The organic phases were combined and washed with 50 mL of satura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com