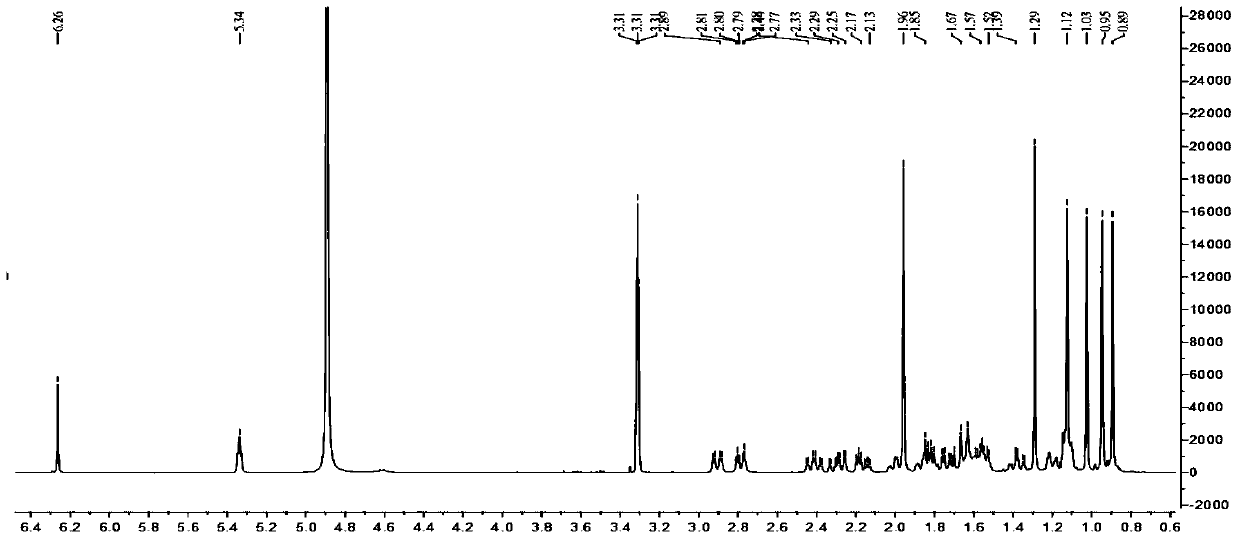
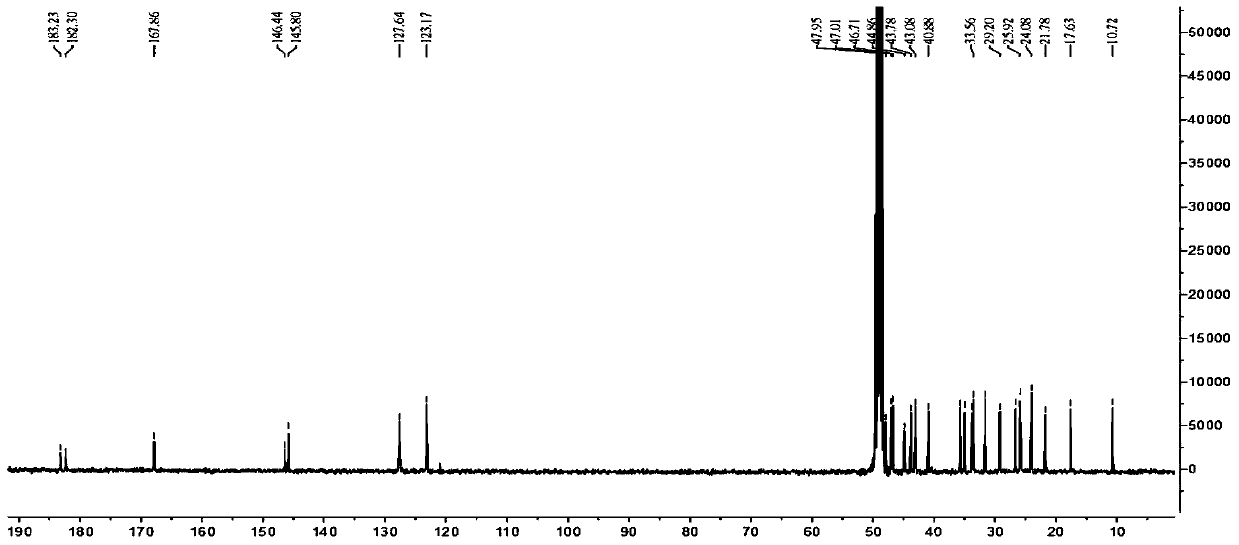
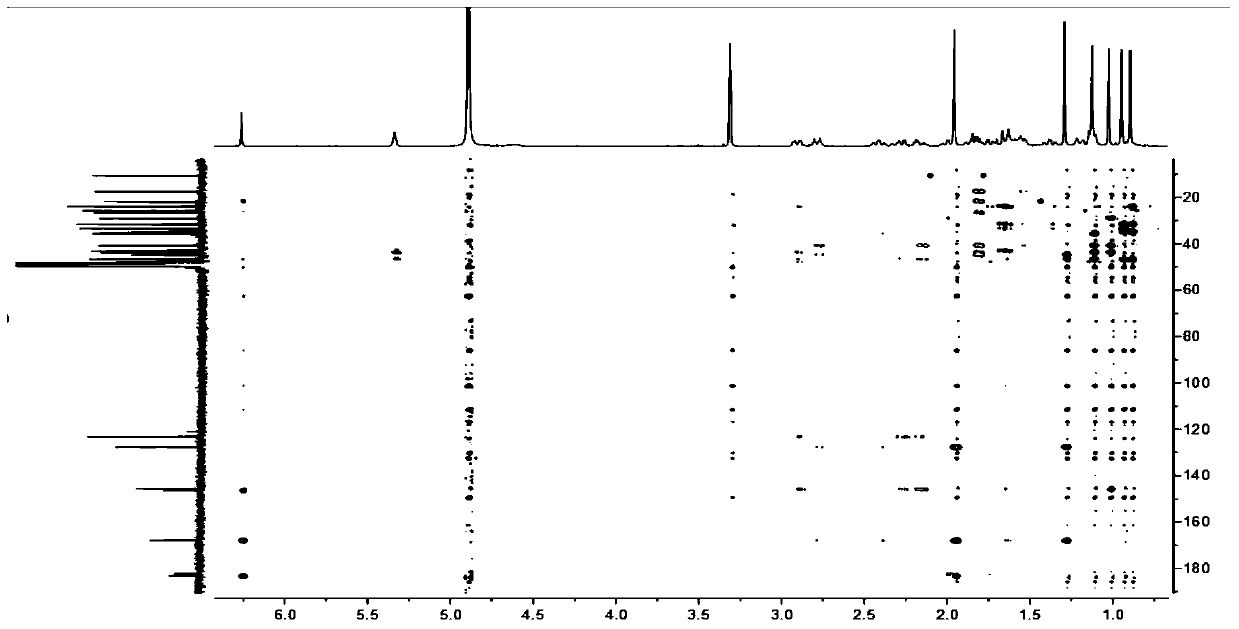
Novel 23-oleanolic acid compound as well as preparation method and application of compound in preparation of glycosidase inhibitor medicine
A technology for reducing oleanberries and compounds, which is applied in the field of natural medicinal chemistry, can solve the problems of insufficient pharmacological activity and ingredients, and achieves the effects of good market prospect, rich material sources and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of 2-hydroxy-3-carbonyl-23-norolean-1,4,12-triene-28-acid in Akebia clover fruit
[0025] 1.1 Instruments and reagents
[0026]The decompression concentration adopts N-1000 rotary evaporator of Tokyo Physical and Chemical Company, CCA-1110 circulating cooling box and SB-1000 electric heating constant temperature water bath; HPLC adopts Japan Shimadzu Company LC-20AT liquid chromatograph, SPD-M20A detection and Shim-PackPRC-ODS chromatographic column (particle size 5μm, pore size 12nm, 250mm×20mm); electrospray mass spectrometry (ESIMS) adopts MDSSCIEXAPI2000LC / MS / MS instrument from Applied Biosystems, USA, and uses methanol as solvent for direct sample determination; 1 HNMR spectrum and 13 The CNMR spectrum was measured using a Bruker DRX-400 nuclear magnetic resonance instrument with tetramethylsilane as an internal standard. The color development method adopts 10% sulfuric acid ethanol solution or sulfuric acid vanillin treatment, and then hea...
Embodiment 2
[0038] Example 2: Preparation of 2-hydroxy-3-carbonyl-23-norolean-1,4,12-triene-28-acid in the stems and leaves of Akebia trifoliata
[0039] 2.1 Instruments and reagents: same as Example 1
[0040] 2.2 Plant source and identification: same as Example 1
[0041] 2.3 Extraction and separation
[0042] The sample (Akebia trifoliate stems and leaves, dry weight 2.0 kg) was crushed and extracted three times with 95% ethanol aqueous solution at room temperature, and the combined filtrate was concentrated under reduced pressure to remove the organic solvent to obtain the crude extract of the total extract. Suspend the crude extract of the total extract in 500ml of water, then extract with an equal volume of petroleum ether, and concentrate the extract under reduced pressure to obtain the total extract of petroleum ether (24g). Dissolve the total extract of petroleum ether with chloroform / methanol (100mL) with a volume ratio of 1:1, add normal phase silica gel (80-100 mesh) and mix...
Embodiment 3
[0044] Take the stems, leaves or fruits of Akebia akebiae, Akebia changxu, Akebia basilicate or Akebia trilobata as samples, and finally purify according to the extraction and separation methods described in Example 1 to obtain pure compounds of formula (I) 2,3 ,20-Trihydroxy-29-norolean-12-ene-28-oic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com