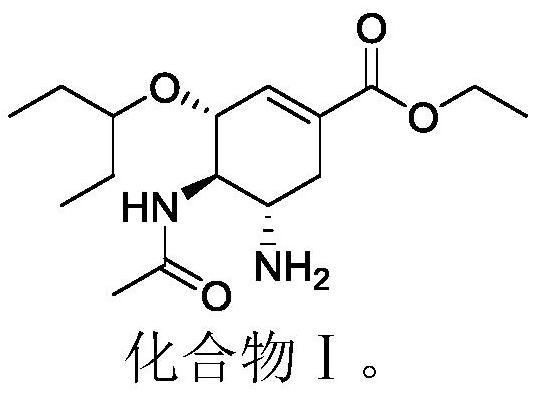
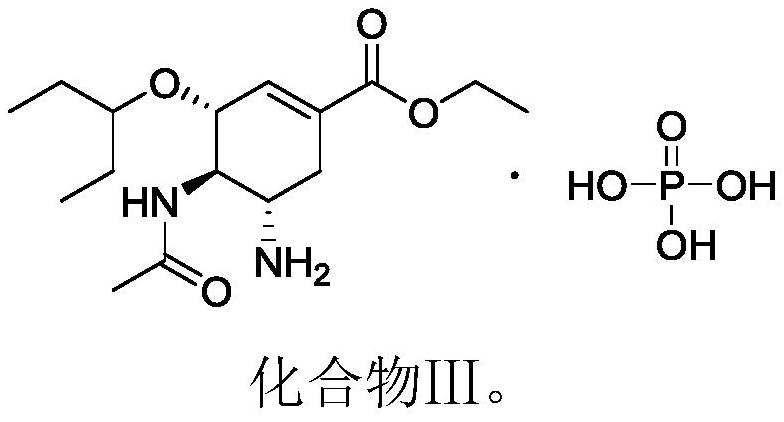
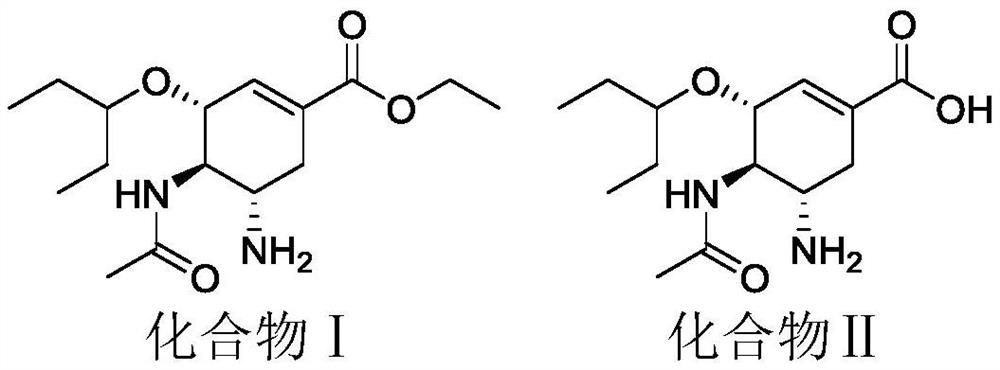
Use of a cyclohexenyl-dl-aspartic acid derivative in the quality control of neuraminidase inhibitor pharmaceutical preparations
A technology of aspartic acid and neuraminidase, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 2
[0021] Compound Preparation Example 2 Preparation and Structure Confirmation of Compound A
[0022] Take 1mmol ethyl (3R,4R,5S)-4-acetylamino-5-amino-3-(pent-3-yloxy)cyclohex-1-ene-1-carboxylate (compound S) and 1.2 Mmol fumaric acid was dissolved in 70% acetonitrile to obtain a saturated solution, and the pH of the solution was adjusted to 9 with 0.1N sodium hydroxide. The resulting solution was stirred in a 70°C water bath for 24 hours. Distilled to dryness under reduced pressure, the resulting solid was washed three times with 0.01N sodium hydroxide, and purified by HPLC to obtain a white powdery solid with a melting point of 88.9°C to 90.2°C. The H-NMR spectrum data of the starting material and the product are shown in Table 2. The HPLC analysis results showed that the retention time of the obtained product in the HPLC measured by the method described in Test Example 1 was 43.985 min.
[0023] Table 2 Proton NMR spectrum data of relevant compounds
[0024]
[0025] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com