23-Norursane triterpene compound and its preparation method and application in the preparation of glycosidase inhibitor drugs
A technology of glycosidase inhibitors and triterpenoids, applied in the field of natural medicinal chemistry, can solve the problems of allergy and anaphylactic shock in patients, and achieve the effects of high safety, abundant sources and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
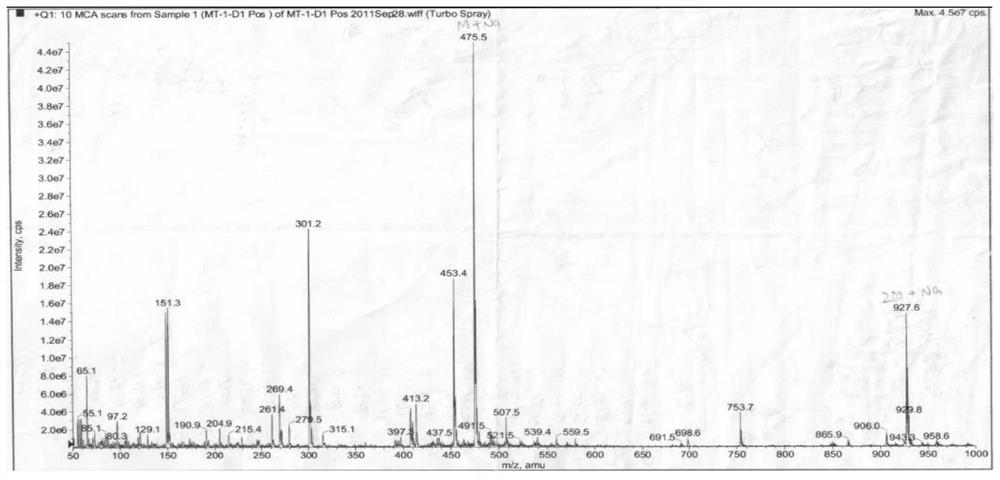
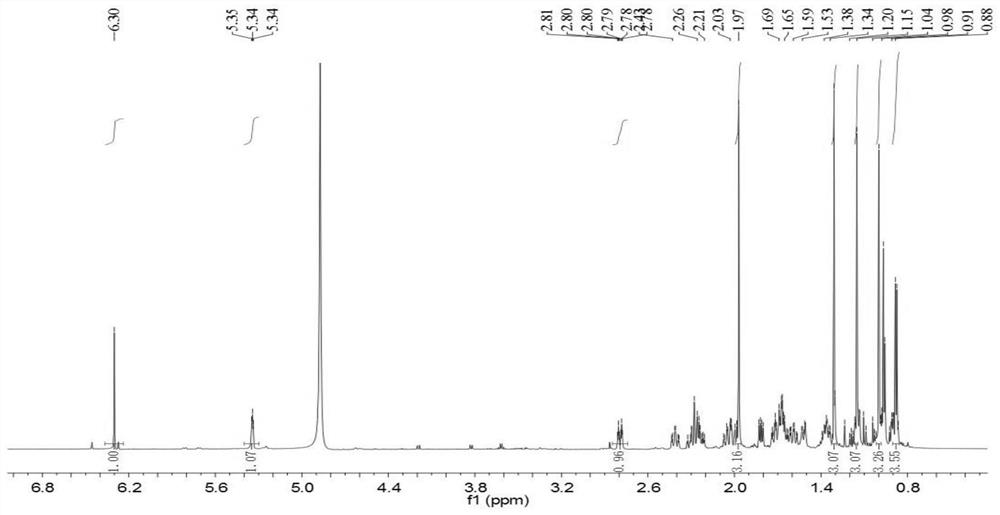
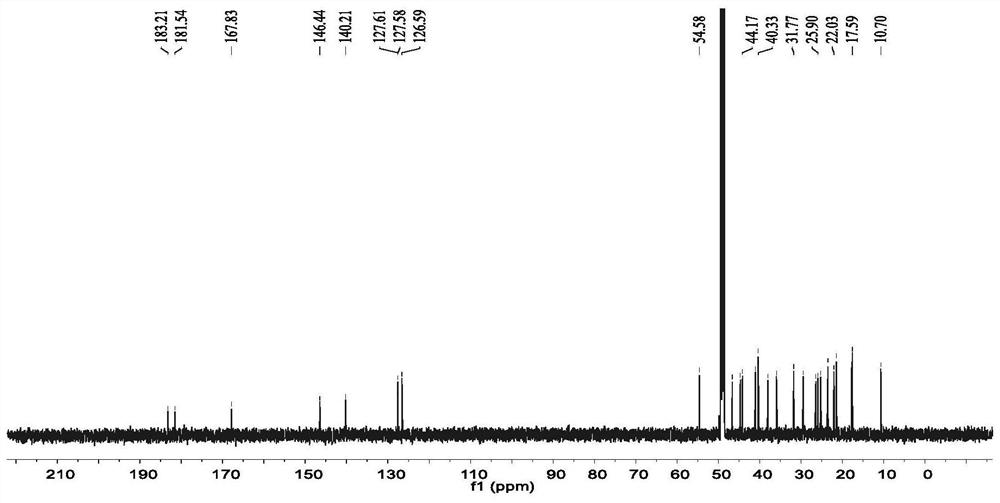
[0024]Example 1: Three-leaf Tongxin New Compound 2-Hydroxy-3-Carbonyl-23-Preparation 1.1 Plant Source of Trichon-28-Acid
[0025]The fruit samples of akebia trifoliata (thumb.) Koidz. The fruit samples were collected from Hunan Province in September 2017.
[0026]1.2 extraction and separation
[0027]Sample (three-herafly fruit dry product, 2.0 kg) Crushing, extract three times with a volume fraction of 95% ethanol solution, combined with filtrate to remove organic solvents to give a total extract. The total dipper was suspended in 500 ml of water, then extracted with an equal volume of petroleum ether, and the extract was concentrated under reduced pressure to obtain a petroleum ether total extract (31 g). The total extract of the petroleum ether was dissolved with a volume ratio of 1: 1 chloroform / methanol (100 mL), and the positive phase silica gel (80-100 mesh) was shaped at a weight ratio of 1: 1.5, and the dried column (200- 300 mesh, 800 grams), dry method, use petroleum ether / ace...
Embodiment 2
[0038]Example 2: Compound 2-Hydroxy-3-Carbonyl-23-Degradation of Umowa-1,4,12-Trivadiene-28-Glycosidase Inhibitory Activity Detection 2.1 Instruments & Reagents
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com