Applications of composition of ligustrazine and mannuronic acid in preparation of medicines for treating thrombotic diseases and nervous system diseases
A technology of mannuronic acid and thrombotic diseases, applied in the field of medicine, to achieve good market application prospects, prevent and treat thrombotic diseases, and strong thrombotic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
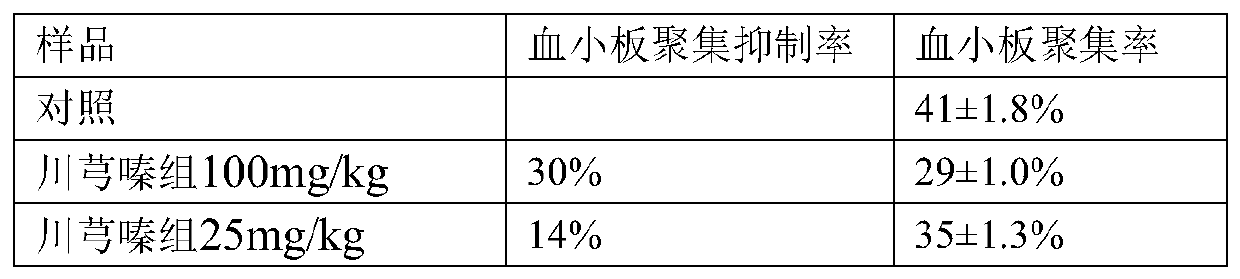
[0022] Embodiment 1: The effect of oral administration of ligustrazine and mannuronic acid on platelet aggregation in experimental rats
[0023] Select SD rats, male, 3-4 weeks old, provided by the Experimental Animal Center of Shandong Province. Experimental animals were divided into 5 groups, 6 in each group, control group, Ligustrazine 100mg / kg; Mannucuronic acid 200mg / kg; kg; intragastric administration; once a day, continuous administration for one week, after the experiment, fix the rats on the operating table, puncture the heart with a needle to take blood, and anticoagulate with sodium citrate at the same time, centrifuge at 1000rpm for 10 minutes, and absorb the supernatant The liquid is platelet-rich plasma PRP; the remaining blood is centrifuged again at 3000rpm for 20 minutes, and the supernatant is platelet-poor plasma PPP. Adjust PRP with PPP Platelet count is 4X10 8 / ml. Adjust to zero with PPP, add PRP to the pen cloud tube, warm bath at 37°C for 10 minutes,...
Embodiment 2
[0028] Embodiment 2: The effect of oral ligustrazine and mannuronic acid on rabbit platelet aggregation
[0029] New Zealand rabbits were selected as experimental animals, weighing 2-3 kg, male, and provided by the Experimental Animal Center of Shandong Province. Experimental animals were divided into 5 groups, 6 in each group, control group, Ligustrazine 100mg / kg; Mannucuronic acid 200mg / kg; kg; intragastric administration; once a day, continuous administration for one week, after the experiment, fix the rabbit supine on the operating table, puncture the heart with a needle to take blood, anticoagulate with sodium citrate, centrifuge at 1000rpm for 10 minutes, and draw up The supernatant is platelet-rich plasma PRP; the remaining blood is centrifuged again at 3000rpm for 20 minutes, and the supernatant is platelet-poor plasma PPP. PRP platelet count was adjusted to 4X108 / ml with PPP. Adjust to zero with PPP, add PRP to the pen cloud tube, warm bath at 37°C for 10 minutes, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com