A class of chromone compounds, their preparation method and their application in the preparation of anti-tumor and enzyme inhibitor drugs
A technology of anti-tumor drugs and compounds, applied in anti-tumor drugs, drug combinations, organic chemistry, etc., can solve the problems of less research and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of sulfur-containing chromone compound oxalicumoneA and compound 1-3
[0050] Preparation method of PDB medium per liter: Mix 200 grams of potatoes, 20 grams of glucose, and 30 grams of sea salt, and dilute to 1L with water. PDB medium was filled into about 300 1000mL Erlenmeyer flasks, each bottle was about 300mL, sterilized by high-pressure steam at 121°C for 25 minutes, and set aside.
[0051] PDA medium configuration method per liter: Mix 200 grams of potatoes, 20 grams of glucose, 30 grams of sea salt, and 20 grams of agar, and dilute to 1L with water. Autoclave at 121°C for 25 minutes and set aside.
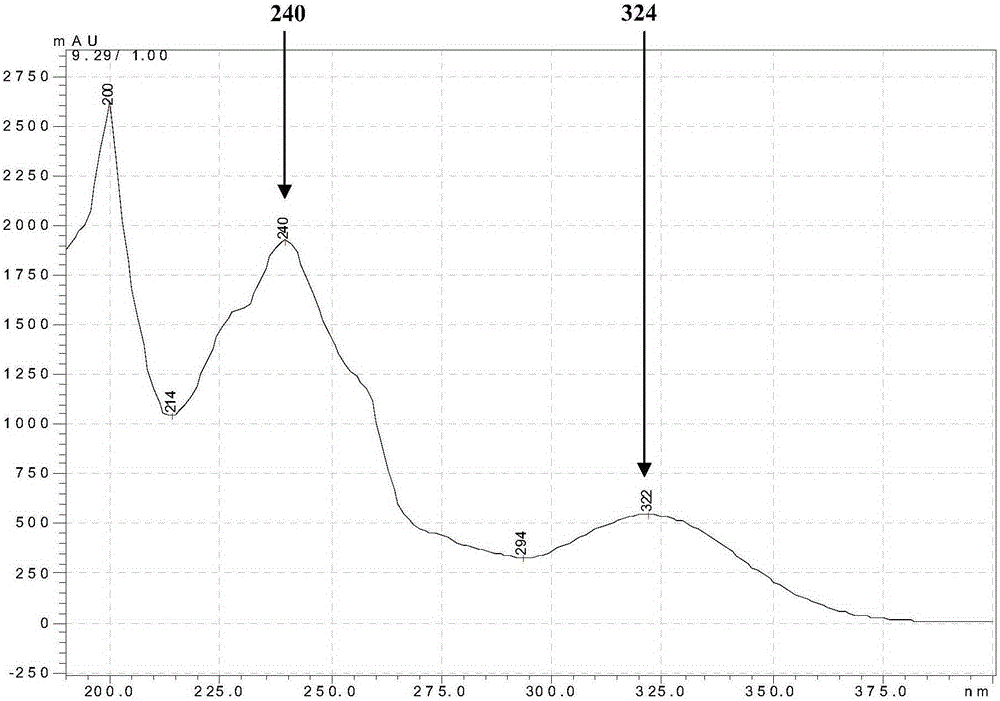
[0052] Use a bamboo stick to pick an appropriate amount of Penicillium oxalicum (Penicillium oxalicum) SCSGAF0023CCTCCNO: M2012507 and inoculate it on the PDA medium, and culture it at 28°C for 3 days to obtain a plate with cultured bacteria, and then use a bamboo stick to pick an appropriate amount of bacteria from the plate Inoculate int...
Embodiment 2
[0070] Embodiment 2: Preparation of sulfur-containing chromone compounds 4-8
[0071] Weigh 2.0 mgoxalicumone A and dissolve it in a 5 mL flask containing 0.5 mL of anhydrous pyridine, add 15 μl of 3,5-bis(trifluoromethyl)benzoyl chloride (BTBC), and then add a small amount of 4-dimethylaminopyridine (DMAP ) as a catalyst, reacted with shaking at 28°C for 10 hours, evaporated the solvent to dryness under reduced pressure, and prepared the crude product by HPLC semi-preparation (MeOH / H 2 O, v / v87.5:22.5, 3ml / min) after washing for 20min, then use MeOH / H 2 O (v / v100:0, 3ml / min) was eluted for 8 min, and the fraction was collected to obtain compound 7. In the same way, when a small amount of BTBC is added (7 μl), compound 6 can be reacted, using high performance liquid phase semi-preparation (MeOH / H 2 (0, v / v80:20, 3ml / min), the peak time of collecting is 17.5min sample obtains. Using the same method, the reaction reagent BTBC was replaced by 4-nitrobenzoyl chloride (NC) and b...
Embodiment 3
[0079] Embodiment 3: Preparation of sulfur-containing chromone compounds 9-10
[0080] Weigh 2.0 mg of compound 1 and dissolve it in 0.5 mL of anhydrous pyridine, add 10 μL of Mosher reagent (R)-2-methoxy-2-trifluoromethylphenylacetyl chloride and 1.0 mg DMAP (as a catalyst) to the solution, at room temperature Reacted for 9 hours. After the solvent was evaporated to dryness under reduced pressure, the crude product was prepared by HPLC semi-preparative (MeOH / H 2 (0, v / v80:20, 3ml / min), and the collected peak time was 18.5min to obtain compound 9.
[0081] The method for preparing compound 10 is similar to that of compound 9, except that the Mosher reagent is replaced by (S)-2-methoxy-2-trifluoromethylphenylacetyl chloride.
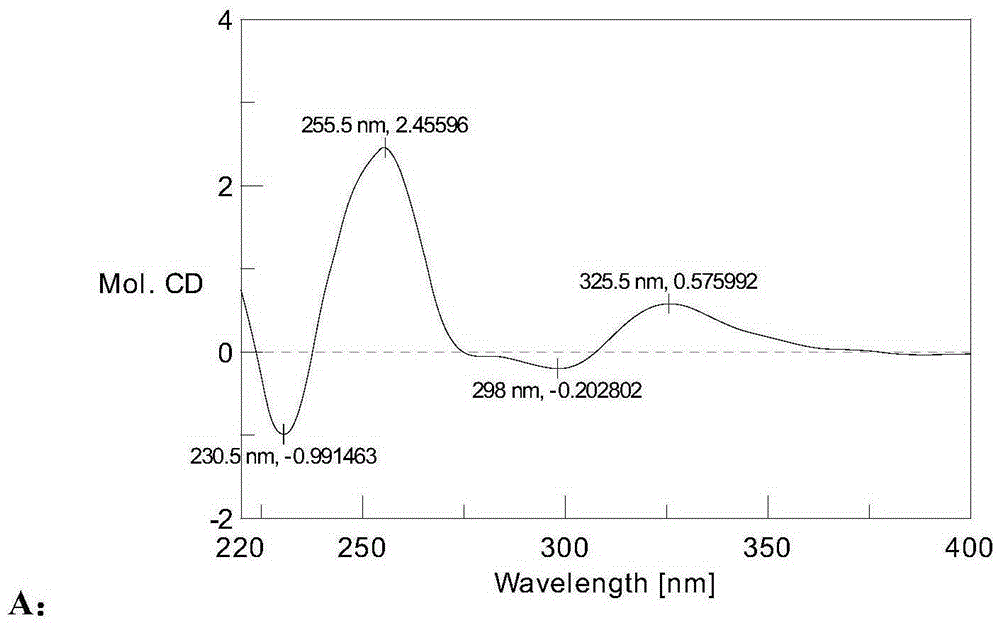
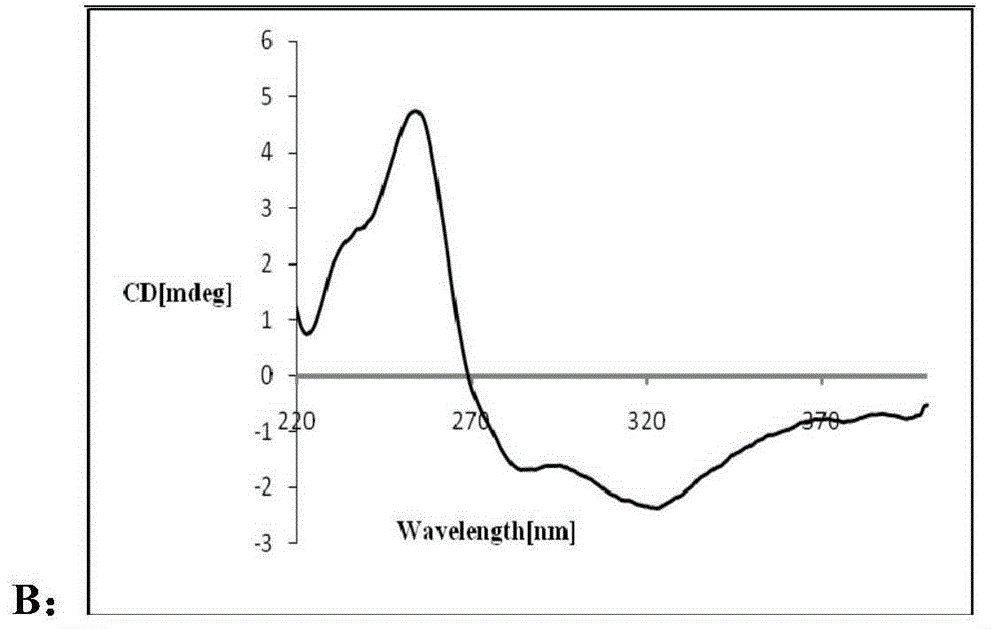
[0082] Compounds 9 and 10 are the Mosher esterification products of compound 1. According to the nuclear magnetic resonance data and comparison with the nuclear magnetic resonance data of compound 1, the structures of compounds 9 and 10 are determined a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com