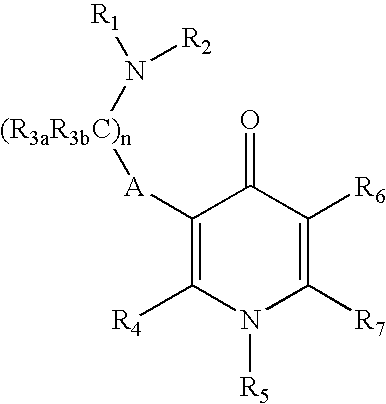
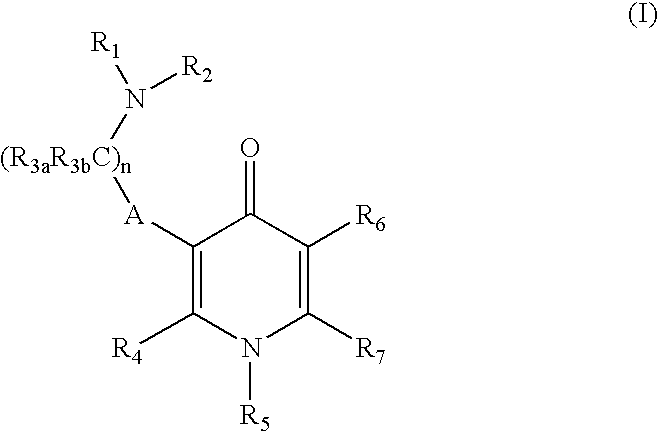
Gonadotropin-releasing hormone receptor antagonists and methods relating thereto
a technology of gonadotropin and receptor antagonists, which is applied in the field of gonadotropin-releasing hormone receptor antagonists, can solve the problems of low bioavailability and adverse side effects of peptidic antagonists with low histamine release properties, and the clinical use of such antagonists, and achieve the effect of preventing or treating bone loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
SYNTHESIS OF 1-(2,6-DIFLUOROBENZYL)-2,6-DIMETHYL-3-BROMO-5-ETHOXYCARBONYL-4-PYRIDONE
[0119]
Step 1A Ethyl-3-(2.6-difluorobenzylamino)crotonate:
[0120] 2,6-Difluorobenzylamine (5.00 g, 35.0 mmol) was added to trimethyl orthoformate (88.0 mL) under N2. The resulting solution was stirred at room temperature for 20 minutes. Ethyl acetoacetate (4.5 mL, 35.0 mmol) was then added dropwise and the resulting mixture stirred for 24 hours (reaction progress monitored by LC / MS). The volatiles were removed under vacuum and the product solidified upon standing. Yield 7.88 g (30.9 mmol, 88%). 1H NMR (CDCl3, 300 MHz): 8.86 (br, 1H), 7.30-7.21 (m, 1H), 6.94-6.81 (m, 2H), 4.48 (s, 2H), 4.07 (q, J=6.9 Hz, 2H), 2.03 (s, 3H), 1.23 (t, J=6.9 Hz, 3H); LRMS m / z 256.1 (M++1).
Step 1B 1-(2.6-Difluorobenzyl)-2,6-dimethyl-3-ethoxycarbonyl-4-pyridone:
[0121] A mixture of ethyl-3-(2,6-difluorobenzylamino)crotonate (2.55 g, 10.0 mmol) and 2,2,6-trimethyl-1,3-dioxin-4-one (1.85 g, 13.0 mmol) in toluene (100 mL) was...
example 2
SYNTHESIS OF 1-(2,6-DIFLUOROBENZYL)-2,6-DIMETHYL-3-(2-FLUORO-3-METHOXYPHENYL)-5-[2-(2-PYRIDYL)ETHYLAMINOMETHYL]-4-PYRIDONE
[0123]
Step 2A 1-(2,6-Difluorobenzyl)-2.6-dimethyl-3-(2-fluoro-3-methoxyphenyl)-5-ethoxycarbonyl-4-pyridone
[0124] 1-(2,6-Difluorobenzyl)-2,6-dimethyl-3-bromo-5-ethoxycarbonyl-4-pyridone (1.5 g, 3.7 mmol) was evacuated in a pressure vessel with 2-fluoro-3-methoxyphenyl boronic acid (764 mg, 4.5 mmol) and tetrakis(triphenylphosphine) palladium (0) (428 mg, 370 μmol). A mixture of benzene / ethanol / 1,2-dimethoxyethane (74 ml in a 10 / 1 / 11 ratio) and barium hydroxide (saturated aqueous, about 0.5 M) was then added under vacuum, the vessel sealed under N2, and the reaction stirred at 90° C. for twelve hours, protected from light. The organic layer was concentrated and purified using flash silica chromatography (EtOAc / hexanes-1 / 4 to MeOH / CH2Cl2-2 / 98), with the product eluting as the third spot. The product was dried as an amber oil (1.12 g, 2.5 mmol, 68%). 1H NMR (CDCl3,...
example 3
SYNTHESIS OF 1-(2,6-DIFLUOROBENZYL)-2,6-DIMETHYL-3-(2-FLUORO-3-METHOXYPHENYL)-5-[2-(2-PYRIDYL)ETHYLAMINOMETHYL]-4-PYRIDONE
[0129]
Step 3A 1-(2.6-Difluorobenzyl)-2.6-dimethyl-3-(2-fluoro-3-methoxyphenyl)-5-[N-{2-(2-pyridyl)ethyl}-N-methylaminomethyl]-4-pyridone
[0130] Sodium triacetoxyborohydride (30 mg, 142 μmol) was added to a stirring solution of 1-(2,6-difluorobenzyl)-2,6-dimethyl-3-(2-fluoro-3-methoxybenzyl)-5-formyl-4-pyridone (20 mg, 50 μmol) and 2-(2-methylaminoethyl)pyridine (0.14 mL, 985 μmol) in dichloromethane (1 mL). The solution stirred overnight at r.t., MeOH (0.5 mL) was added and the solution concentrated, taken up in acetonitrile, filtered and purified using a Gilson Prep-HPLC system to give the product as a TFA salt in 34% yield.
[0131] The following compounds are synthesized according to above procedure.
TABLE 3No.R6R1R2N—MS (MH+)3-12-F-3-MeOPh2-PyCH2CH2NMe5223-22-F-3-MeOPhPhCH2NMe5073-32-F-3-MeOPh2-PyCH2NMe5083-42-F-3-MeOPh2-PyCH2NEt5223-52-F-3-MeOPh(2-PyCH2)2N58...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com