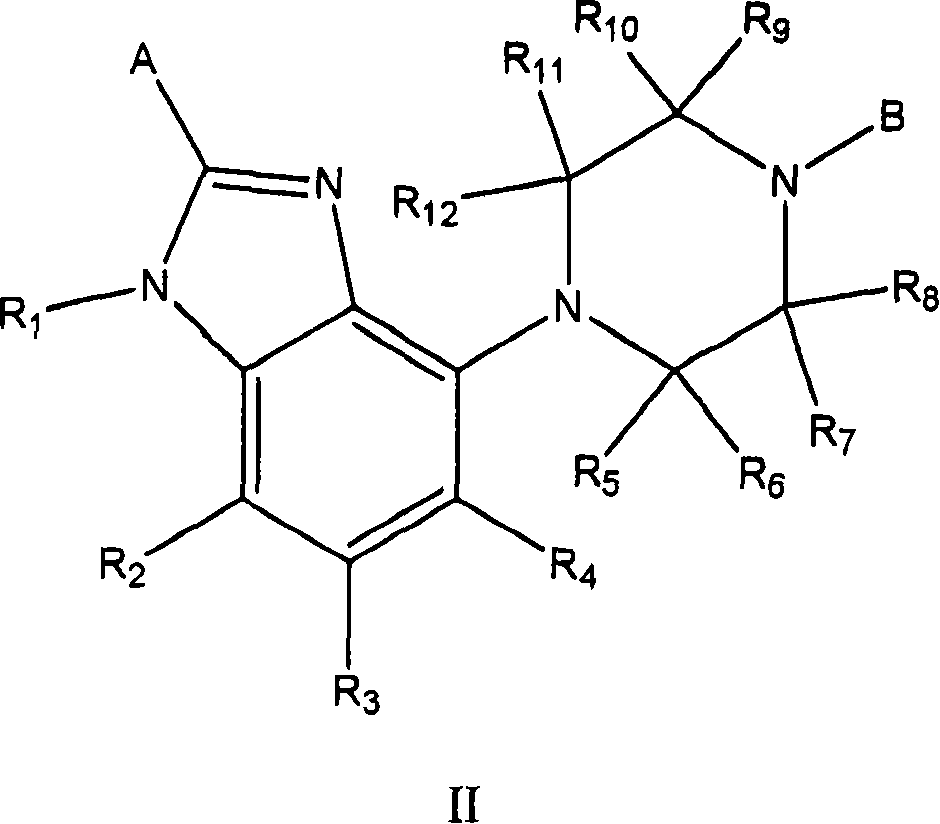
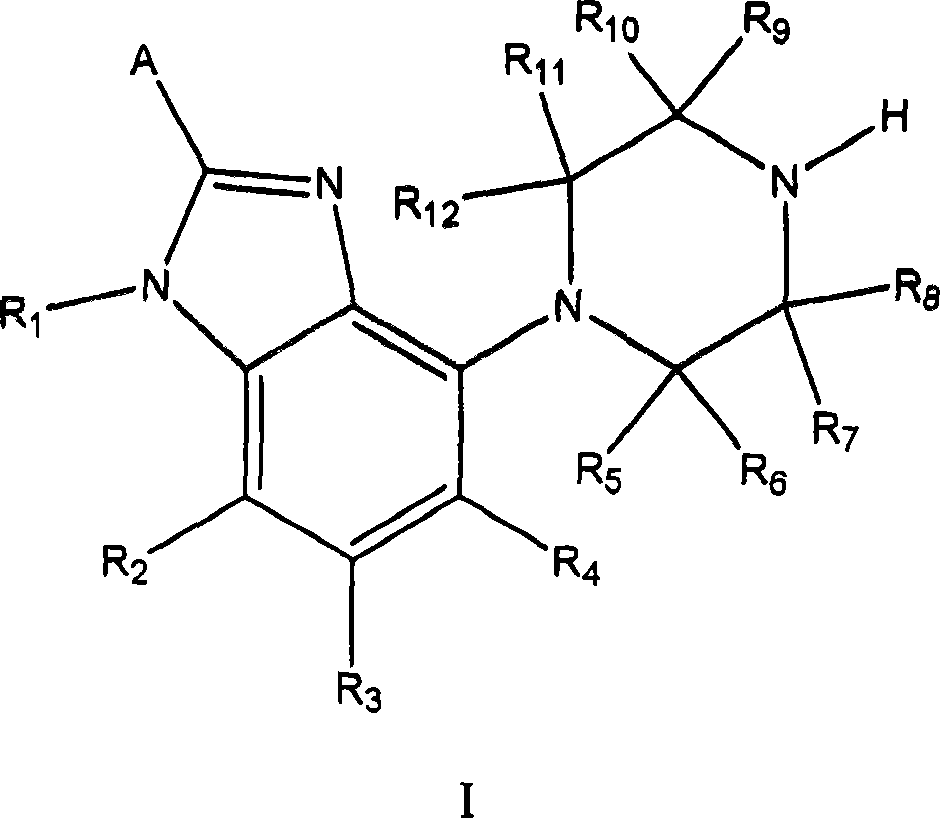
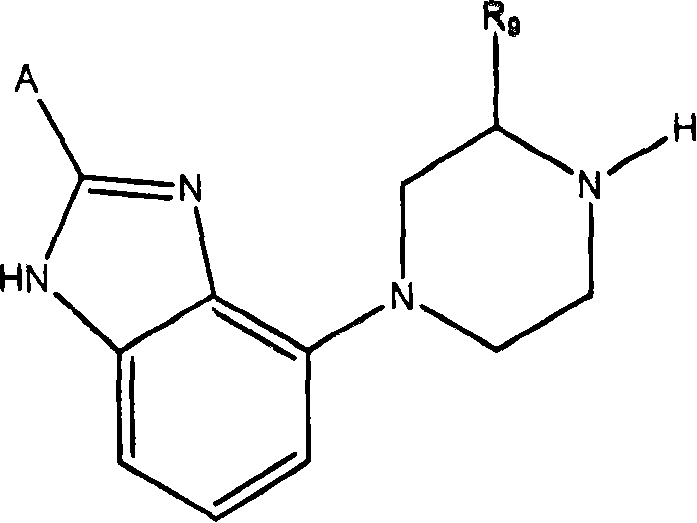
Processes for preparing gonadotropin releasing hormone receptor antagonists
A technology of alkyl and compound, which is applied in the field of preparation of gonadotropin-releasing hormone (GnRH) receptor antagonist, and can solve problems such as not being able to be used orally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] LCMS analysis was performed on an open-frame Agilent chromatograph with UV and mass detectors, 5 cm C18 column, 5 min 95% water to 95% MeCN gradient. HPLC analysis was performed on a Waters liquid chromatograph, 15 cm C18 column, 20 min elution with a 10 min gradient of 95% water to 95% MeCN (0.05% TFA) and a flow rate of 1 mL / min.
N-(2-bromophenyl)-4-tert-butylbenzamide
[0113] 2-Bromoaniline (98%, Oakwood 005347, 214 g, 1.25 moles), sodium bicarbonate (270 g, 3.2 moles), THF (500 mL) and water (500 mL) were charged into a device equipped with an overhead stirrer, thermometer and Add a 500 ml addition funnel to a 5 L round bottom flask. A solution of tert-butylbenzoyl chloride (>98%, Fluka 19660, 250 mL, 1.37 mol) in 250 mL THF was charged to the addition funnel and added slowly (over 25 minutes) to the stirred reaction mixture in the flask (Without external heating or cooling, a slight warming was observed and the temperature in the flask rose to 35°C).
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com