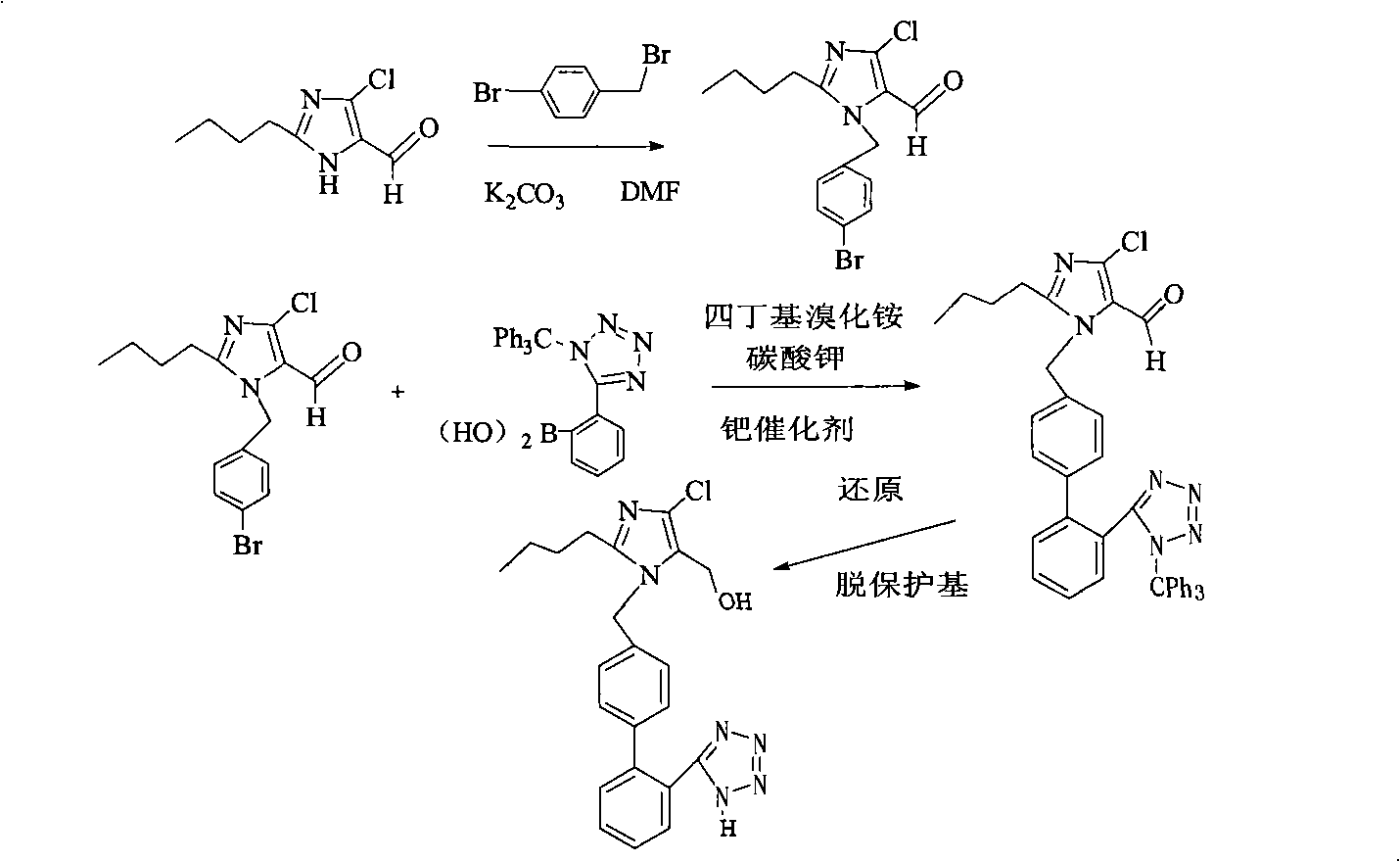
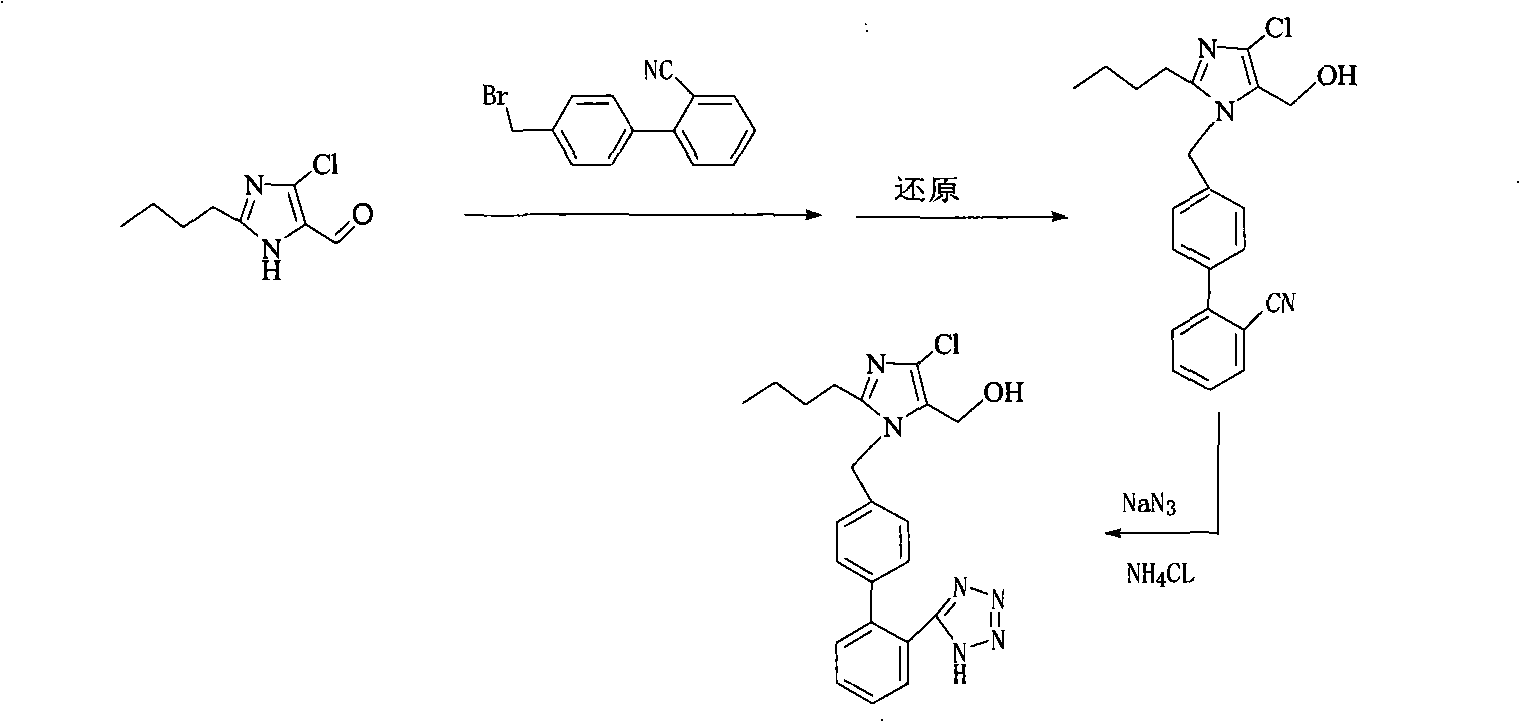
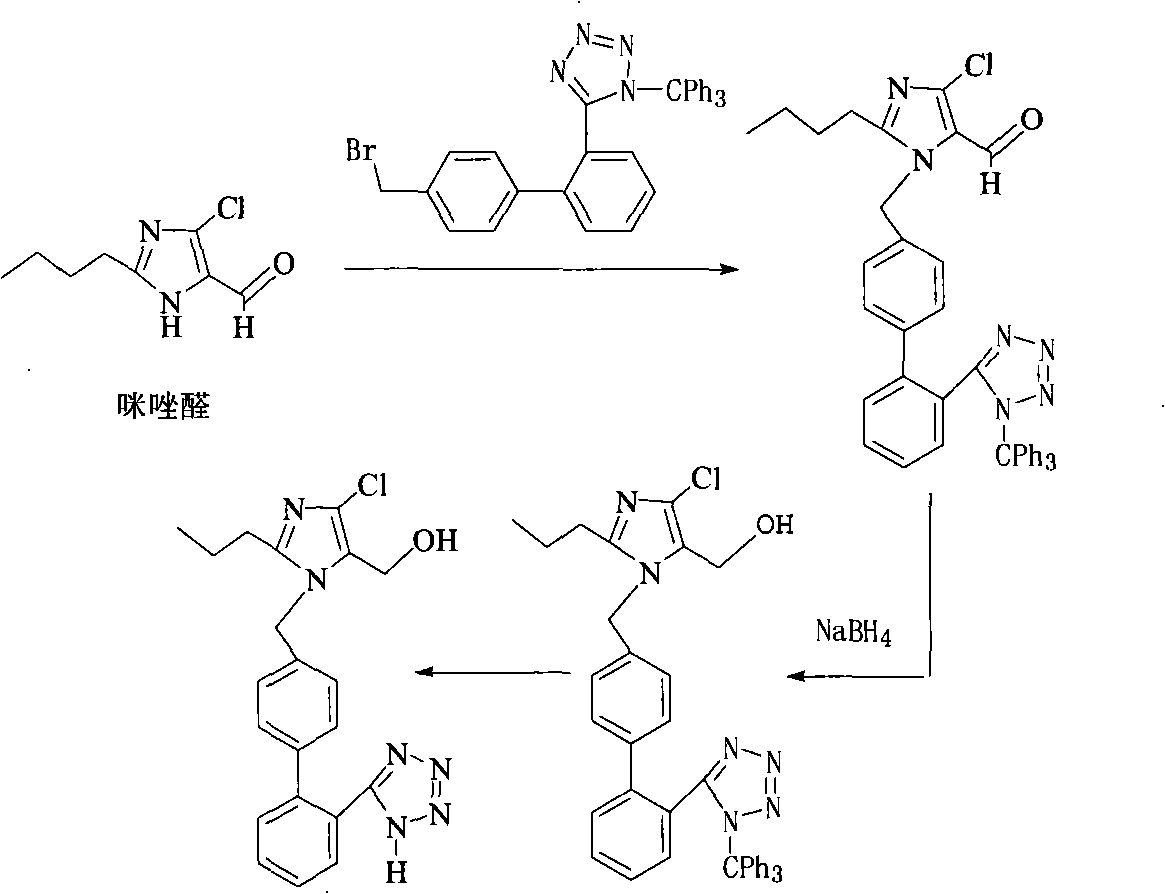
Preparation of losartan
A solvent and formyl technology, applied in the field of medicinal chemistry, can solve the problems of cumbersome operation, low yield, and many steps, and achieve the effects of high product quality, easy product, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 2-butyl-4-chloro-5-formyl-1-[[(2'-(1-trityl-tetrazol-5-yl)-biphenyl-4-)methyl ] the synthesis of imidazole (structural formula IV)
[0029] In a 1L three-necked flask, add 33.5g (0.179mol) 2-butyl-4-chloro-5-hydroxymethylimidazole, 100g (0.179mol) 5-(4-bromomethylbiphenyl-2-yl)- 1-trityl-1H-tetrazole, 300ml DMF, 47g (0.340mol) of potassium carbonate as a base, the reaction was carried out at room temperature for 24 hours, the reaction was completed, the reaction solution was poured into 600ml of water, and a white solid was obtained by suction filtration. Wash with 100ml of water to obtain 2-butyl-4-chloro-5-formyl-1-[[(2'-(1-trityl-tetrazol-5-yl)-biphenyl-4-)methyl Base] imidazole crude product, the crude product does not need to be treated, and the next step reaction is carried out directly, and the yield is 89%.
[0030] 1 HNMR ((CDCl3): 0.85(t, 3H), 1.23-1.32(sextet, 2H), 1.60-1.68(m, 2H), 2.50(t, 2H), 5.45(s, 2H), 6.83(d, 2H ), 6.91-6.93(m, 6H), 7.10...
Embodiment 2
[0031] Example 2: 2-butyl-4-chloro-5-formyl-1-[[(2'-(1-H-tetrazol-5-yl)-biphenyl-4-)methyl]imidazole ( Synthesis of structural formula III)
[0032] 82g (0.123mol) 2-butyl-4-chloro-5-formyl-1-[2'-(1-trityl-tetrazolyl-5-)biphenyl-4-]methyl Imidazole, methanol 420ml, stirred, then added 3.4N HCl40ml (0.136mol) in 10min, stirred overnight at room temperature, added 10N NaOH, adjusted to pH = 13, evaporated ethanol (420mL), added 206mL deionized water. The aqueous layer was washed twice with 2×100ml of toluene, and the separated aqueous layer was slowly dripped with 3.4N hydrochloric acid to pH=3.0, added with 200ml of dichloromethane and stirred for 1 hour, left to stand for liquid separation, and the organic layer was dried with magnesium sulfate. The solvent was evaporated under reduced pressure to obtain the crude product, which was separated by column chromatography, using ethyl acetate:petroleum ether (volume ratio) 3:1 as eluent, to obtain 2-butyl-4-chloro-5-formyl- Pure ...
Embodiment 3
[0034] Example 3: 2-butyl-4-chloro-5-formyl-1-[[(2'-(1-H-tetrazol-5-yl)-biphenyl-4-)methyl]imidazole ( Synthesis of structural formula III)
[0035] Other conditions remain unchanged, the solvent is changed from methanol to tetrahydrofuran, and the yield is 35%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com