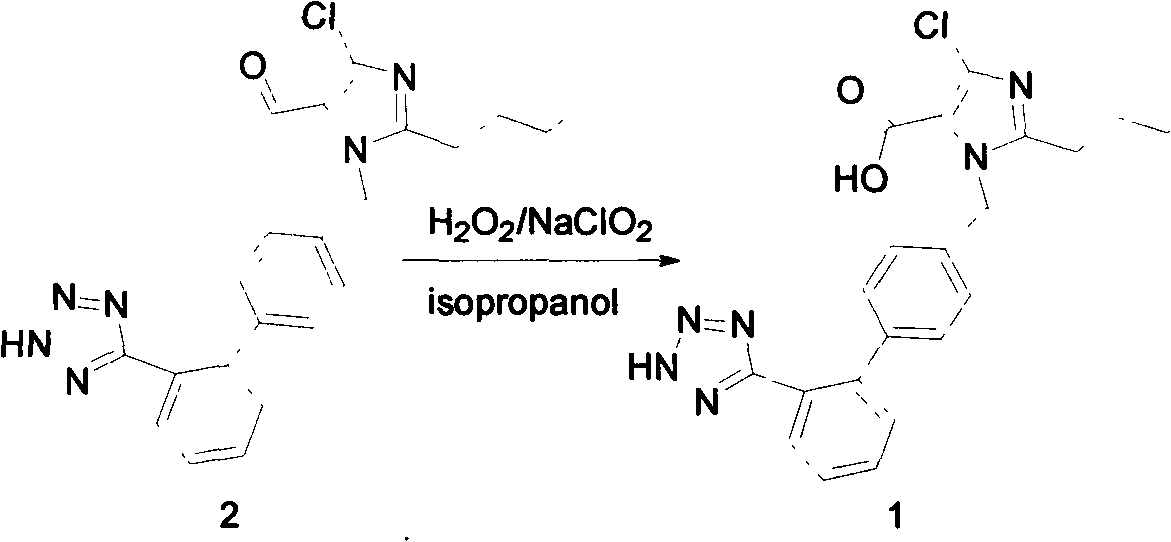Preparation method of 5-losartan carbonate
A technology of losartan and carboxylic acid, which is applied in the field of derivatives of antihypertensive drug losartan, and can solve problems such as unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
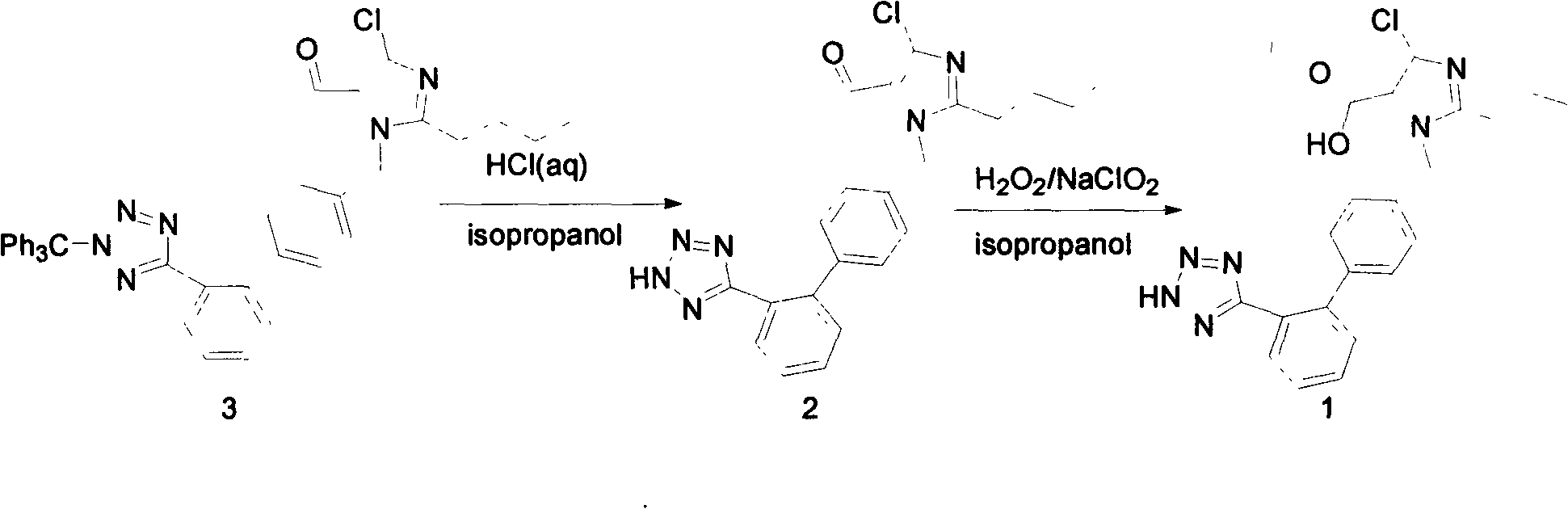
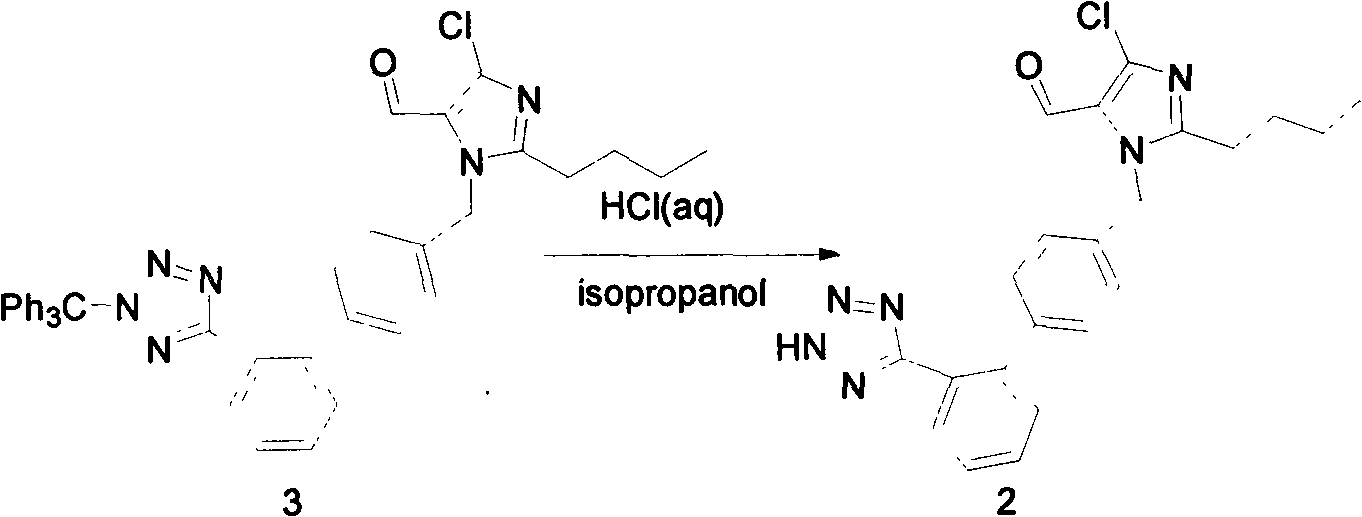
[0014] Preparation of 2-butyl-4-chloro-5-formyl-1-{[2'-2(1-H-tetrazol-5-yl)-biphenyl-4]-methyl}imidazole
[0015]
[0016] Add formula (3) compound (22.5g, 34.2mmol) and isopropanol (150ml) in the 250ml three-neck flask equipped with reflux condenser and dropping funnel, stir mechanically, heat up to 50 ℃, solid does not dissolve, 0.5 h Add 3.4N hydrochloric acid (24ml) dropwise, the solid dissolves, the solution is yellow-green, keep the temperature at 50°C and stir the reaction, TLC (PE:EA=2:1) tracks the reaction progress, after 5h it shows that the basic reaction of the raw materials is complete. Stop heating and stirring, cool and stand overnight. There were colorless transparent particles precipitated at the bottom of the bottle, filtered, and the solid particles were confirmed to be trityl alcohol by comparing with the HPLC spectrum of the standard sample. The filtrate was poured into 500ml of water, stirred for 0.5h, and a white solid was precipitated. After filt...
Embodiment 2
[0019] Preparation of 5-carboxylate losartan
[0020]
[0021] Add compound 4 (3.0 g, 6.93 mmol) and 50 ml of isopropanol to a 100 mL three-necked flask, heat to 40° C. and stir to dissolve the compound 4 solid, then cool down to 5° C., and add 30% hydrogen peroxide (1.17 ml). In addition, sodium chlorite (1.84g) was dissolved in water (14.12ml), and the prepared sodium chlorite solution was added to the three-necked flask in batches within 2 hours at 5°C, and TLC (DCM:MeOH=10:1) Following the progress of the reaction, after 5 hours, it was shown that there was basically no raw material in the reaction. Stop stirring and let stand overnight. Pour the reaction solution into 500ml of water, stir to precipitate white solids, add 10N / L sodium hydroxide solution to the suspension, when the pH is 13, the liquid becomes clear, filter to remove insoluble impurities, pour the filtrate back into the beaker, and quickly Add concentrated hydrochloric acid dropwise under stirring cond...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com