Method of synthesizing losartan and losartan intermediates
A technology of losartan and intermediates, which is applied in the field of preparation of drugs and intermediates, can solve the problems of difficult industrialization, difficult operation, and high cost, and achieve the effects of simple operation, safety assurance, and cost saving of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: preparation formula 1 compound losartan
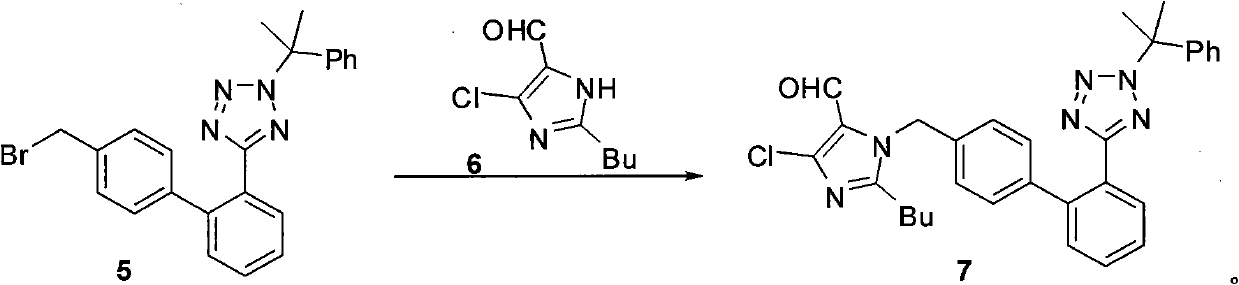
[0044] Step a: Preparation of compound of formula 7: 2-butyl-4-chloro-5-formyl-1-[[2'-[2-(1-methyl-1-phenylethyl)tetrazole-5- Base] biphenyl-4-yl] methyl] imidazole
[0045] Add 17.4g (0.04mol) of the compound of formula 5 and 7.5g (0.04mol) of the compound of formula 6 into 135g of N,N-dimethylformamide, cool to 0-5°C, add 7.0g (0.05mol) of potassium carbonate, and React at 0-5°C for 8 hours, then raise the temperature to 30°C and react for 9-12 hours. The reaction solution was poured into 300g of ice-water mixture, a pale yellowish white solid was precipitated, filtered, the filter cake was dissolved in 300mL of dichloromethane, washed with 150mL×2 water, the organic layer was dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure. 26.0 g of oil was obtained, which was purified by silica gel column chromatography, eluting with ethyl acetate / n-hexane, and 19.8...
Embodiment 2
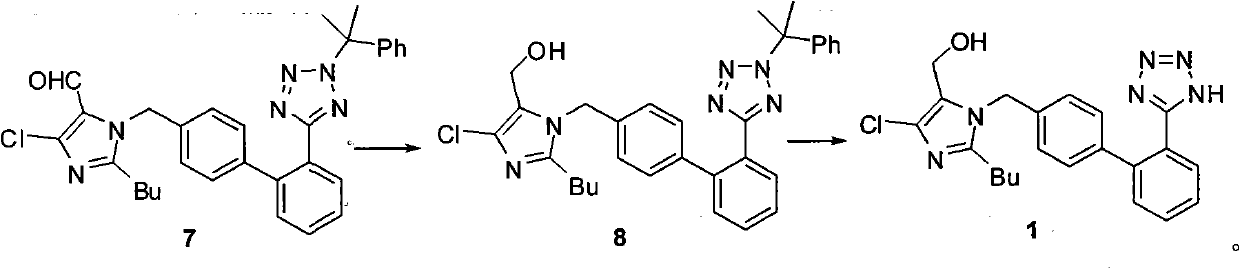
[0060] Embodiment 2: One-pot preparation formula 1 compound losartan:
[0061] 17.4g (0.04mol) formula 5 compound and 7.5g (0.04mol) formula 6 compound add 150mL toluene, 6.4g (0.08mol) 50% sodium hydroxide aqueous solution, 1.6g (0.002mol) tetrabutylammonium bromide, heating React at 90°C for 5 hours. The reaction solution was cooled to 0-5°C, 1.8g (0.048mol) of sodium borohydride was added, and the reaction was carried out at 0-5°C for 3 hours. Separate the layers, wash the organic layer with 150mL×2 water, cool to 0-5°C, add 5% hydrochloric acid dropwise to adjust the pH to 3, separate the water layer, add 90g 36% hydrochloric acid to the organic layer, and react at 30-35°C for 12 hours . Cool to 0-5°C, add 200mL of water dropwise, separate the layers, wash the organic layer with 150mL×2 water, cool to 0-5°C, a solid precipitates, stir for 1 hour, and filter to obtain 15.7g of a light yellow glass-like solid, which is washed by After recrystallization, 13.6 g of white ...
Embodiment 3
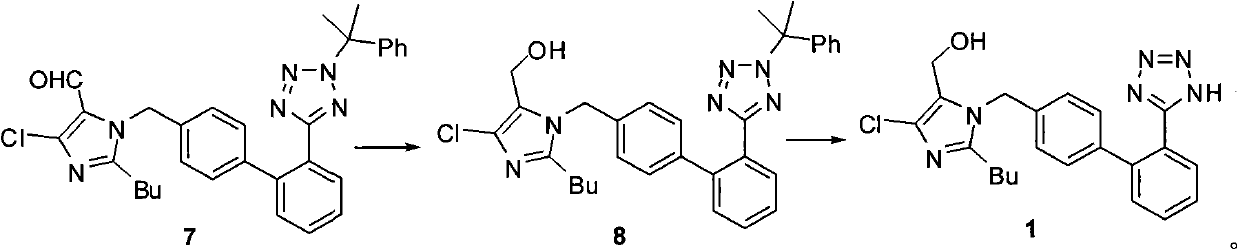
[0063] Embodiment 3: preparation formula 1 compound losartan:
[0064] Step a: Preparation of compound of formula 9: 2-butyl-4-chloro-5-formyl-1-[[2'-(tetrazol-5-yl)biphenyl-4-yl]methyl]imidazole
[0065] 21.6g (0.04mol) of the compound of formula 7 (from step a in Example 1), was added with 200mL of isopropanol and 90g of 36% hydrochloric acid, and reacted at 30-35°C for 12 hours. Cool to 0-5°C, add 200mL of purified water dropwise, then add dropwise 5% sodium hydroxide solution to adjust the pH to 3, add this mixture dropwise to 3000mL of 0.001mol / L hydrochloric acid, precipitate solids, filter to obtain shallow 19.1 g of yellow solid was recrystallized to obtain 15.4 g of white powdery solid with a yield of 91.7%. Mp 153-154°C.
[0066] 1 H-NMR (DMSO-d 6 , 500MHz) δ: 16.307 (brs, 1H, CN 4 H), 9.696(s, 1H, CHO), 7.678(t, J=7.0Hz, 2H, ArH), 7.579(t, J=7.5Hz, 1H, ArH), 7.524(d, J=7.5Hz, 1H , ArH), 7.133-7.046 (m, 4H, ArH), 5.604 (s, 2H, NCH 2 Ar), 2.641(t, J=7.5Hz, 2H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com