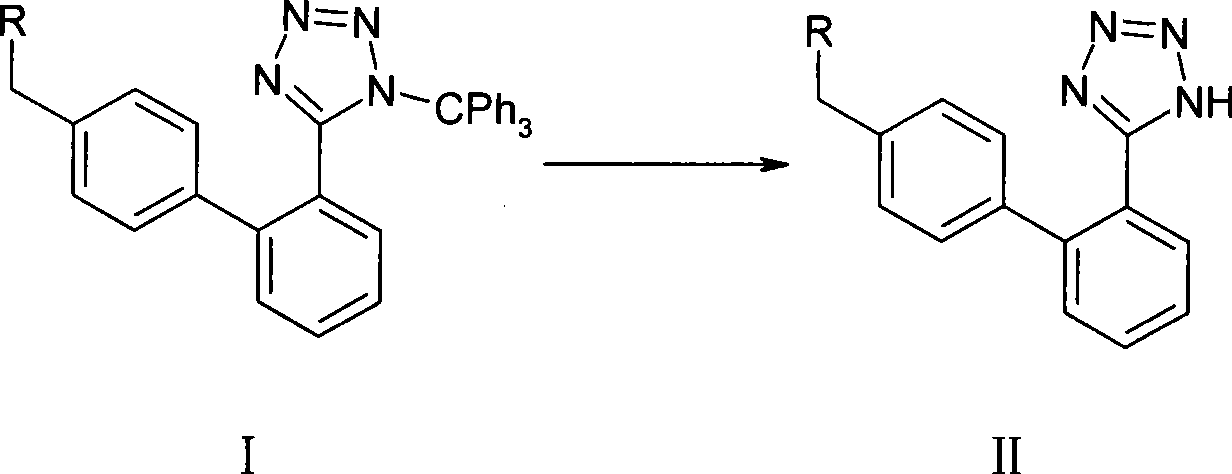
Method for removing trityl group of 1-trityl-5-(1,1'-biphenylyl-2 yl)-1H-tetrazole compound
An azole compound, trityl technology, applied in the field of pharmaceutical synthesis, can solve the problems of easy generation of impurities, high reaction temperature, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
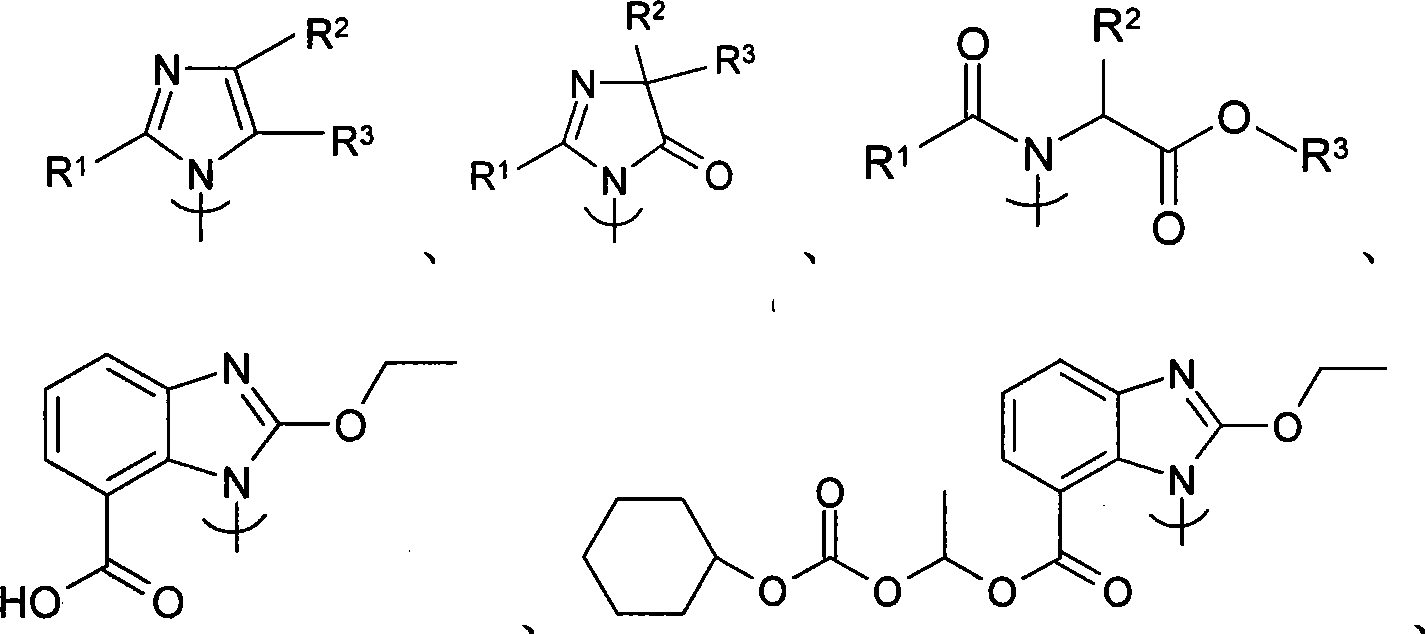
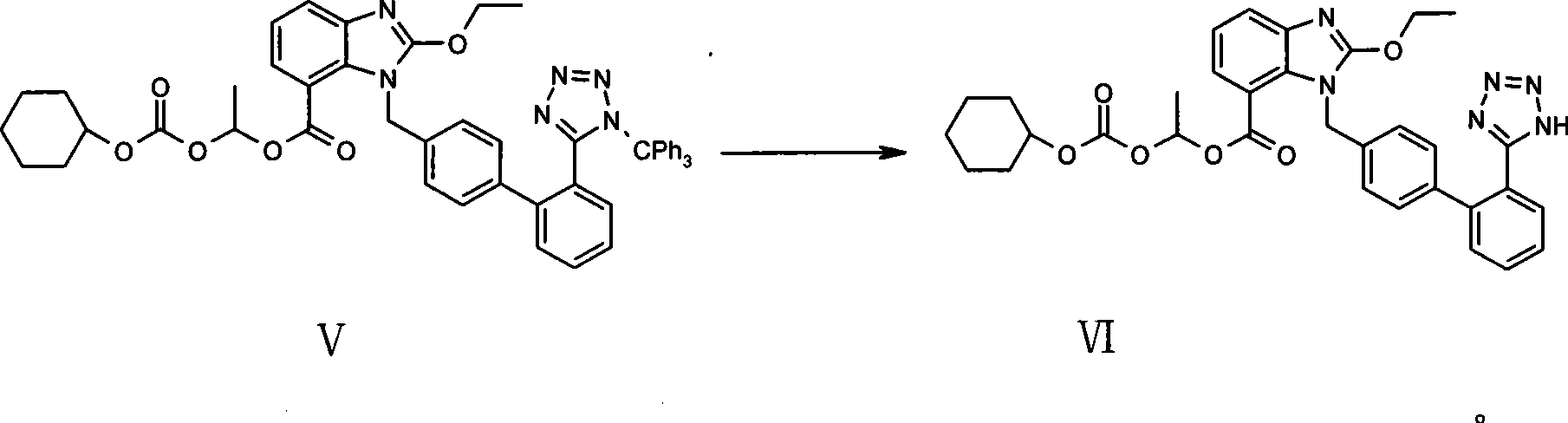
[0046] 1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-[[2`-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]benzo Imidazole-7-carboxylate (candesartan cilexetil, VI)
[0047] Put 1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-[[2`-(N-triphenyl Methyl-1H-tetrazol-5-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylate (trityl candesartan cilexetil, V) 10g, dichloromethane 20ml, methanol 40ml (moisture is 0.23%, KF detection, the same below), stir to dissolve. Acetyl chloride 1ml was added, and the reaction was stirred. After the reaction was finished, 10% potassium bicarbonate solution was added dropwise to adjust the pH value to neutral, and the mixture was left to stand and separated into layers. The aqueous layer was extracted once with 20 ml of dichloromethane. The dichloromethane layers were combined, dried over anhydrous magnesium sulfate, and concentrated to dryness under vacuum. Add 16ml of acetone and 32ml of n-hexane to the residue, stir to precipitate crystals, and filter with ...
example 2
[0049] 1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-[[2`-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]benzo Imidazole-7-carboxylate (candesartan cilexetil, VI)
[0050] Referring to Example 1, 1ml of acetyl chloride was replaced by 1ml of benzoyl chloride, yield 83.9%.
example 3
[0052] 1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-[[2`-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]benzo Imidazole-7-carboxylate (candesartan cilexetil, VI)
[0053] Referring to Example 1, 1ml of acetyl chloride was replaced by 1ml of chloroacetyl chloride, and the yield was 89.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com