Method for recovering valsartan racemate
A technology of valsartan racemate and recovery method, which is applied to the recovery field of valsartan racemate, can solve problems such as being unfavorable to environmental protection, waste resources and the like, and achieve environmental protection, good product quality and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of valsartan devaleryl product and n-valeric acid
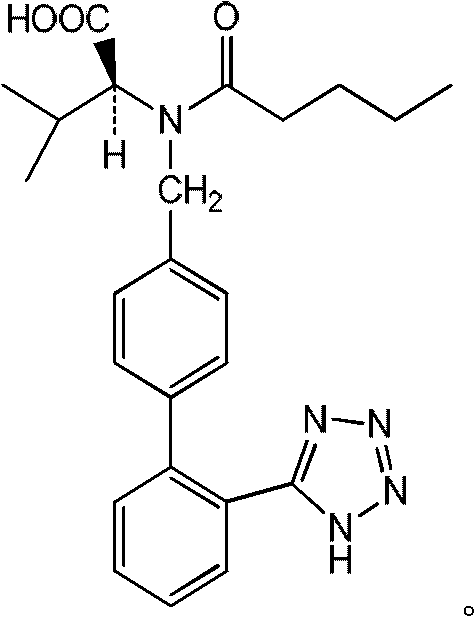
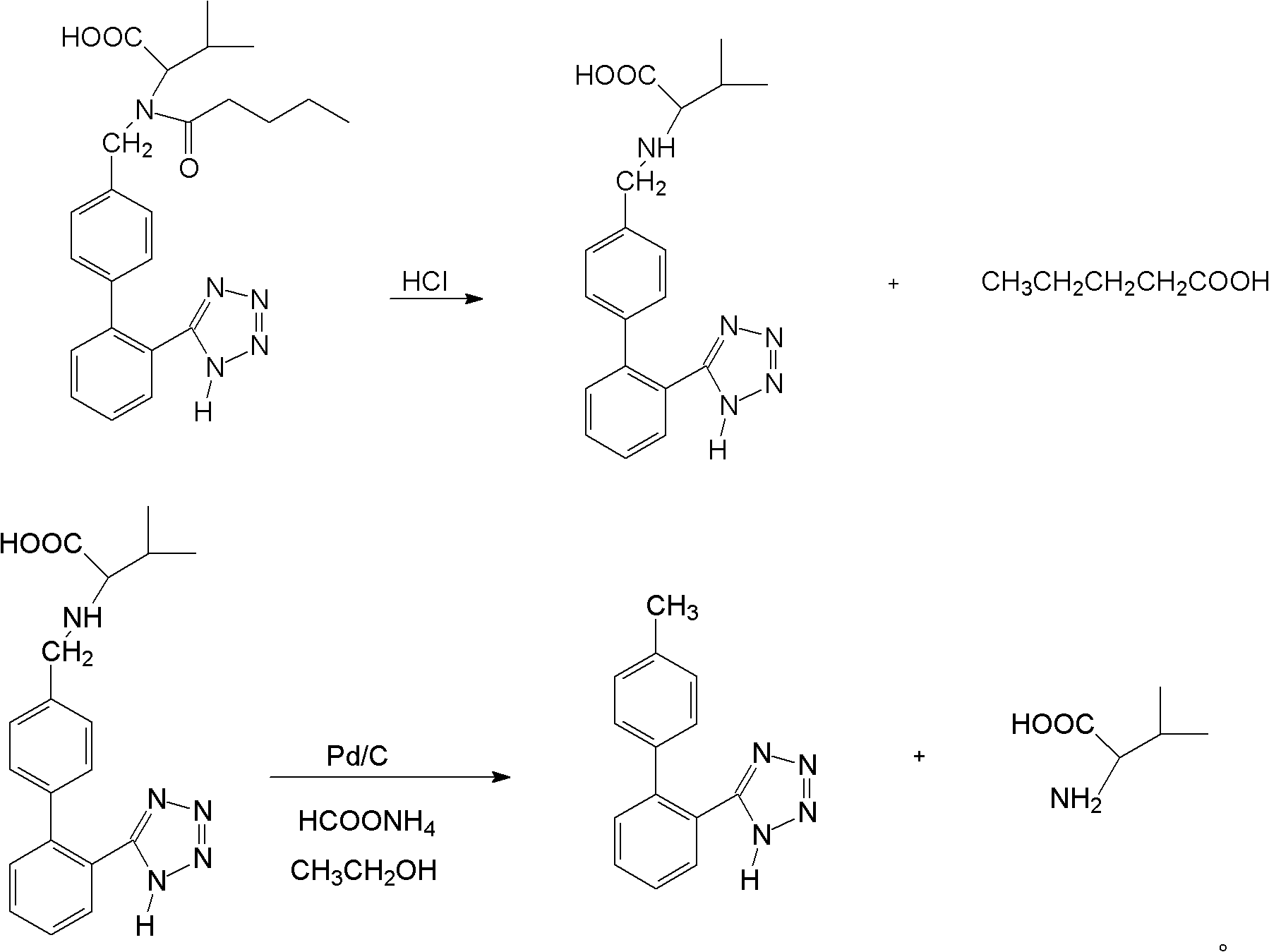
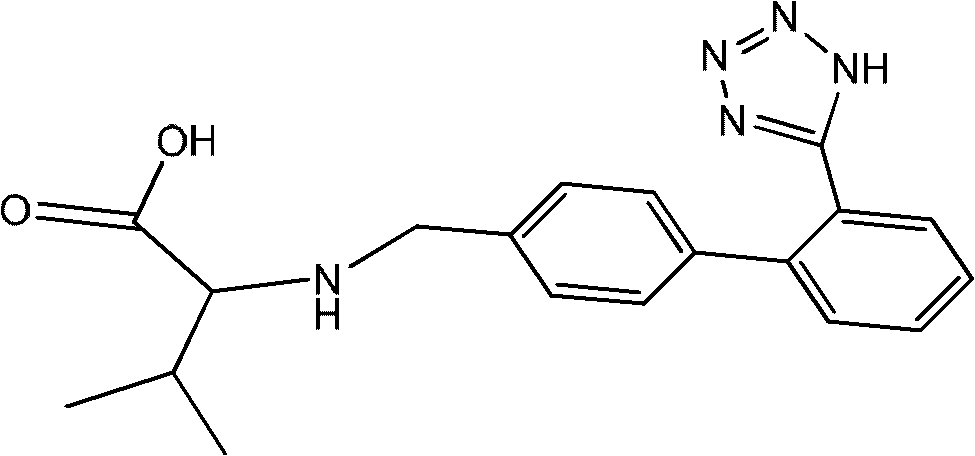
[0033] Get 952g of ethyl acetate liquid of valsartan racemate and join in the reaction bottle of 2L, be heated up to 85 ℃ and steam ethyl acetate, when there is no distillate, obtain 582g (1.34mol) valsartan racemate, 463g of hydrochloric acid aqueous solution with a mass percentage concentration of 24% (including 3.04mol of hydrochloric acid) was added dropwise at heat preservation, and the solution was refluxed at 95° C. for 4 hours after dropping, cooled, and suction filtered. Recover the dry solvent from the filtrate under reduced pressure at 70°C to obtain product a: n-valeric acid, with a molar yield of 98%; add the filter cake to a 1000ml reaction flask, add 300ml of ice water, stir for about 30 minutes, and filter with suction. The filter cake was washed with ice water to weakly acidic pH=6.5, and dried to obtain the valsartan devaleryl product with a molar yield of 98%. The conversion ra...
Embodiment 2
[0045] Example 2 Preparation of valsartan devaleryl product and n-valeric acid
[0046] Get 952g of ethyl acetate liquid of valsartan racemate and join in the reaction bottle of 2L, be heated up to 85 ℃ and steam ethyl acetate, when there is no distillate, obtain 582g (1.34mol) valsartan racemate, 679g of hydrochloric acid aqueous solution with a mass percentage concentration of 36% (including 6.69 mol of hydrochloric acid) was added dropwise at heat preservation, and the solution was refluxed at 100°C for 4 hours after dropping, cooled, and suction filtered. Recover the dry solvent from the filtrate under reduced pressure at 70°C to obtain the product a: n-valeric acid, with a molar yield of 92%; add the filter cake to a 1000ml reaction flask, add 300ml of ice water, stir for about 30 minutes, and filter with suction. The filter cake was washed with ice water to weakly acidic pH=6.0, and dried to obtain valsartan devaleryl product with a molar yield of 92%. The conversion ra...
Embodiment 3
[0058] Example 3 Preparation of valsartan devaleryl product and n-valeric acid
[0059] Take 952g of ethyl acetate solution of valsartan racemate and add it to a 2L reaction flask, heat up to 85°C to distill the ethyl acetate, and when there is no distillate, 582g (1.34mol) of valsartan racemate is obtained 458 g (4.01 mol of hydrochloric acid) was added dropwise with a mass percent concentration of 32% hydrochloric acid solution at an insulated temperature, refluxed at 98° C. for 4 h after dropping, cooled, and suction filtered. Recover the dry solvent from the filtrate under reduced pressure at 70°C to obtain product a: n-valeric acid, with a molar yield of 94%; add the filter cake to a 1000ml reaction flask, add 300ml of ice water, stir for about 30 minutes, and filter with suction. The filter cake was washed with ice water to weakly acidic pH=6.8, and dried to obtain valsartan devaleryl product with a molar yield of 94%. The conversion rate was 98%.
[0060] Confirmation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com