Losartan preparation method
A technology for the preparation process and compound, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of side reactions, affecting the quality of the final product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
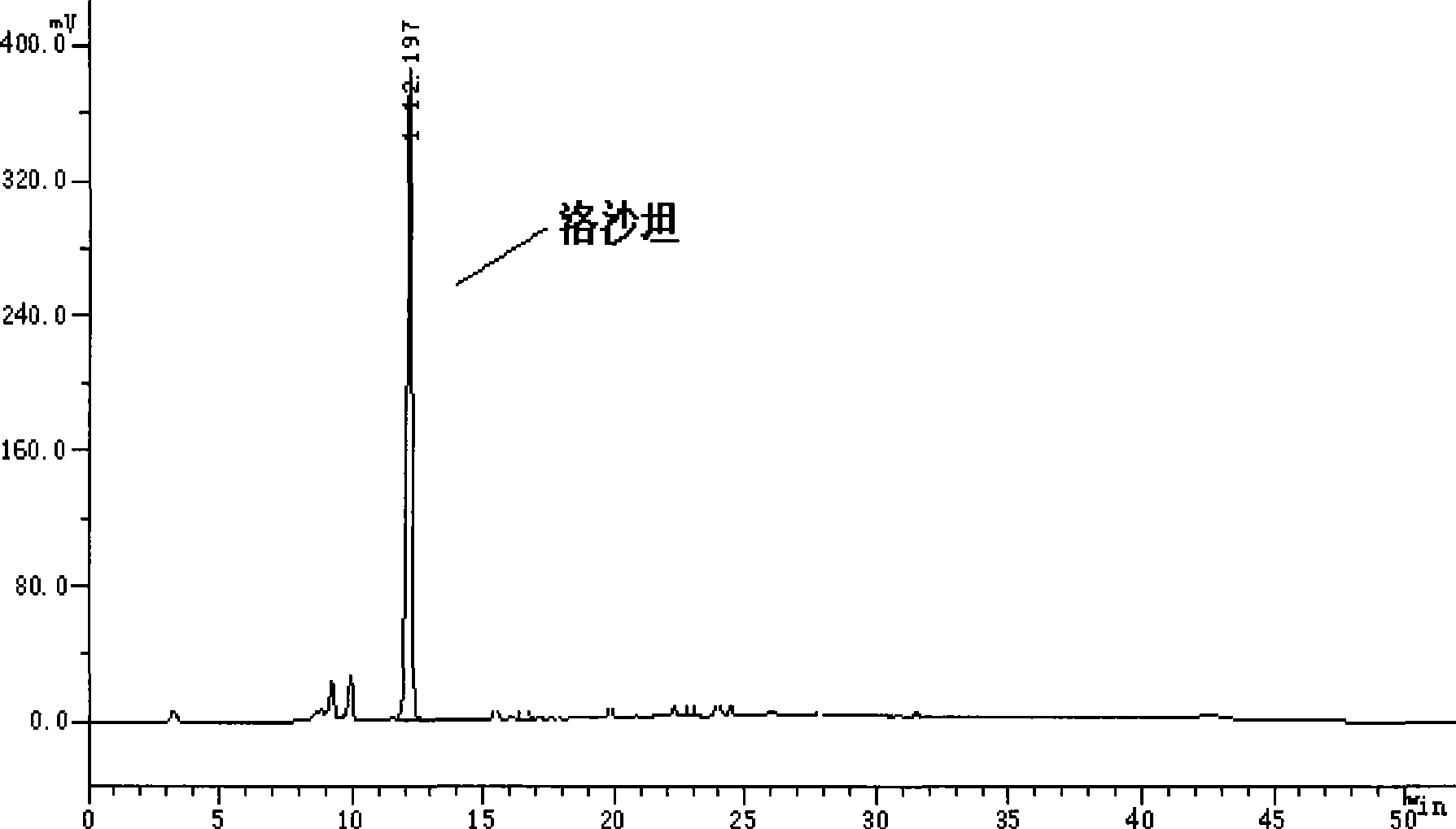
[0015] 32g 2-butyl-4-chloro-5-hydroxymethyl-1-[2'-(1-trityl-tetrazolyl-5-)biphenyl-4-]methylimidazole, 80ml 2 - dodecyl alcohol, then add 3 g of 20% dilute hydrochloric acid, and stir at room temperature for 24 hours to obtain. The liquid phase tracking spectrum of the finished product is shown in figure 1
[0016] current technology:
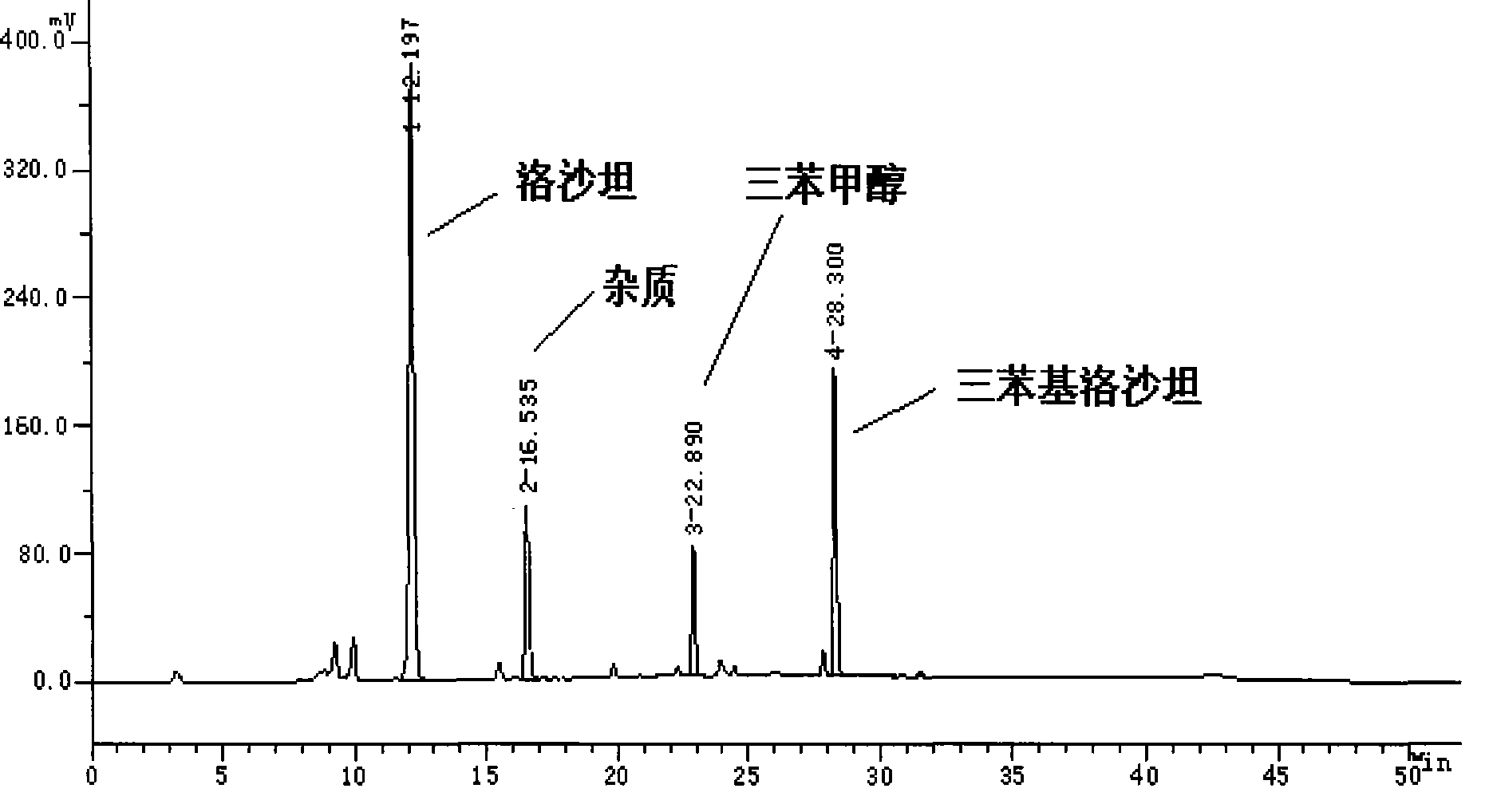
[0017] 32g 2-butyl-4-chloro-5-hydroxymethyl-1-[2'-(1-trityl-tetrazolyl-5-)biphenyl-4-]methylimidazole, 80ml Methanol, then add 3g of 20% dilute hydrochloric acid, stir at room temperature for 24 hours, the liquid phase tracking spectrum of the finished product is shown in figure 2
[0018] Synthesis and structure identification of impurities
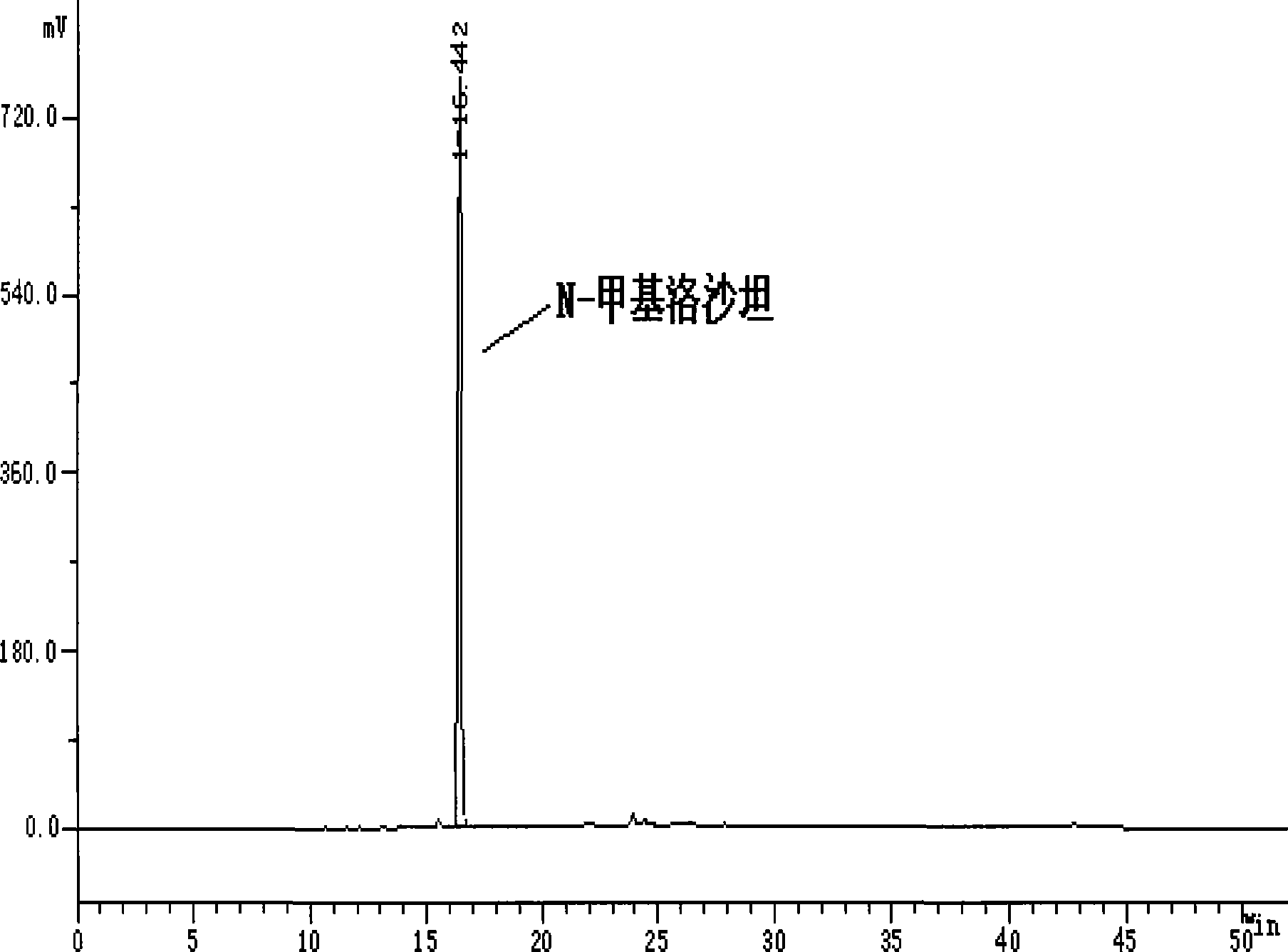
[0019] 10 g of losartan, 50 ml of DMF, 7.8 g of potassium carbonate, and 1.5 ml of methyl iodide were stirred at room temperature for 24 hours to obtain N-methyl losartan with a yield of 23%.
[0020] 1 HNMR (CDCl3): 0.9(t, 3H), 1.2-1.4(m, 2H), 1.5-1.7(m, 2H), 2.6(t, 2H), 3.3(s, 3H), 4.5(s, 2H) ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com